Chemicals
Showing 1201–1350 of 41137 results
-
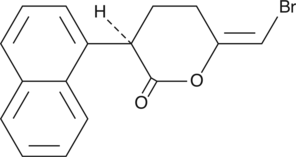
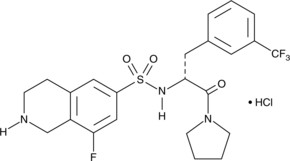
The phospholipases are an extensive family of lipid hydrolases that function in cell signaling, digestion, membrane remodeling, and as venom components.{6940} The calcium-independent phospholipases (iPLA2) are a PLA2 subfamily closely associated with the release of arachidonic acid in response to physiologic stimuli. (R)-Bromoenol lactone ((R)-BEL) is an irreversible, chiral, mechanism-based inhibitor of calcium-independent phospholipase γ (iPLA2γ). Unlike (S)-BEL, (R)-BEL does not inhibit iPLA2β except at high doses of 20-30 µM.{12978} (R)-BEL inhibits human recombinant iPLA2γ with an IC50 of approximately 0.6 µM.
Brand:CaymanSKU:10006800 - 1 mgAvailable on backorder
The phospholipases are an extensive family of lipid hydrolases that function in cell signaling, digestion, membrane remodeling, and as venom components.{6940} The calcium-independent phospholipases (iPLA2) are a PLA2 subfamily closely associated with the release of arachidonic acid in response to physiologic stimuli. (R)-Bromoenol lactone ((R)-BEL) is an irreversible, chiral, mechanism-based inhibitor of calcium-independent phospholipase γ (iPLA2γ). Unlike (S)-BEL, (R)-BEL does not inhibit iPLA2β except at high doses of 20-30 µM.{12978} (R)-BEL inhibits human recombinant iPLA2γ with an IC50 of approximately 0.6 µM.
Brand:CaymanSKU:10006800 - 10 mgAvailable on backorder
The phospholipases are an extensive family of lipid hydrolases that function in cell signaling, digestion, membrane remodeling, and as venom components.{6940} The calcium-independent phospholipases (iPLA2) are a PLA2 subfamily closely associated with the release of arachidonic acid in response to physiologic stimuli. (R)-Bromoenol lactone ((R)-BEL) is an irreversible, chiral, mechanism-based inhibitor of calcium-independent phospholipase γ (iPLA2γ). Unlike (S)-BEL, (R)-BEL does not inhibit iPLA2β except at high doses of 20-30 µM.{12978} (R)-BEL inhibits human recombinant iPLA2γ with an IC50 of approximately 0.6 µM.
Brand:CaymanSKU:10006800 - 5 mgAvailable on backorder
The phospholipases are an extensive family of lipid hydrolases that function in cell signaling, digestion, membrane remodeling, and as venom components.{6940} The calcium-independent phospholipases (iPLA2) are a PLA2 subfamily closely associated with the release of arachidonic acid in response to physiologic stimuli. (R)-Bromoenol lactone ((R)-BEL) is an irreversible, chiral, mechanism-based inhibitor of calcium-independent phospholipase γ (iPLA2γ). Unlike (S)-BEL, (R)-BEL does not inhibit iPLA2β except at high doses of 20-30 µM.{12978} (R)-BEL inhibits human recombinant iPLA2γ with an IC50 of approximately 0.6 µM.
Brand:CaymanSKU:10006800 - 500 µgAvailable on backorder
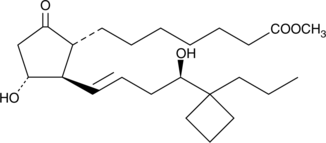
Butaprost is a structural analog of prostaglandin E2 (PGE2) with good selectivity for the EP2 receptor subtype. Butaprost has frequently been used to pharmacologically define the EP receptor expression profile of various human and animal tissues and cells.{3317,1752} Serious confusion as to the structure of butaprost was generated by Gardiner in 1986,{3326} when he reported that the epimer of butaprost showing this selective activity was the C-16 (R)-epimer (See reference {3326} and Note). Butaprost binds with about 1/10 the affinity of PGE2 to the recombinant murine EP2 receptor, and does not bind appreciably to any of the other murine EP receptors or DP, FP, IP, or TP receptors.{6640} The pharmacology of (R)-butaprost has not been carefully studied, but it is generally considered to be the less active epimer.{3178} (NOTE: In the Gardiner paper in the 1986 British Journal of Pharmacology, butaprost appears on page 46 where it is given the name TR 4979. The structure as drawn is incorrect, in that the author was using and referring to the more active C-16 epimer, which is actually 16(S). The structure on page 46 shows the structure as 16(R). It was not until the late 1990’s that careful studies both in the US and Japan correctly identified the actual configuration of C-16 in the compound called butaprost is 16(S).){3326}
Brand:CaymanSKU:-Butaprost is a structural analog of prostaglandin E2 (PGE2) with good selectivity for the EP2 receptor subtype. Butaprost has frequently been used to pharmacologically define the EP receptor expression profile of various human and animal tissues and cells.{3317,1752} Serious confusion as to the structure of butaprost was generated by Gardiner in 1986,{3326} when he reported that the epimer of butaprost showing this selective activity was the C-16 (R)-epimer (See reference {3326} and Note). Butaprost binds with about 1/10 the affinity of PGE2 to the recombinant murine EP2 receptor, and does not bind appreciably to any of the other murine EP receptors or DP, FP, IP, or TP receptors.{6640} The pharmacology of (R)-butaprost has not been carefully studied, but it is generally considered to be the less active epimer.{3178} (NOTE: In the Gardiner paper in the 1986 British Journal of Pharmacology, butaprost appears on page 46 where it is given the name TR 4979. The structure as drawn is incorrect, in that the author was using and referring to the more active C-16 epimer, which is actually 16(S). The structure on page 46 shows the structure as 16(R). It was not until the late 1990’s that careful studies both in the US and Japan correctly identified the actual configuration of C-16 in the compound called butaprost is 16(S).){3326}
Brand:CaymanSKU:-Butaprost is a structural analog of prostaglandin E2 (PGE2) with good selectivity for the EP2 receptor subtype. Butaprost has frequently been used to pharmacologically define the EP receptor expression profile of various human and animal tissues and cells.{3317,1752} Serious confusion as to the structure of butaprost was generated by Gardiner in 1986,{3326} when he reported that the epimer of butaprost showing this selective activity was the C-16 (R)-epimer (See reference {3326} and Note). Butaprost binds with about 1/10 the affinity of PGE2 to the recombinant murine EP2 receptor, and does not bind appreciably to any of the other murine EP receptors or DP, FP, IP, or TP receptors.{6640} The pharmacology of (R)-butaprost has not been carefully studied, but it is generally considered to be the less active epimer.{3178} (NOTE: In the Gardiner paper in the 1986 British Journal of Pharmacology, butaprost appears on page 46 where it is given the name TR 4979. The structure as drawn is incorrect, in that the author was using and referring to the more active C-16 epimer, which is actually 16(S). The structure on page 46 shows the structure as 16(R). It was not until the late 1990’s that careful studies both in the US and Japan correctly identified the actual configuration of C-16 in the compound called butaprost is 16(S).){3326}
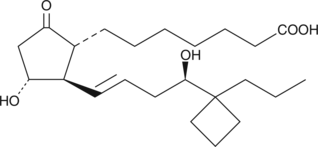
Brand:CaymanSKU:-Butaprost is a structural analog of prostaglandin E2 (PGE2) with good selectivity for the EP2 receptor subtype. Butaprost has frequently been used to pharmacologically define the EP receptor expression profile of various human and animal tissues and cells.{3317} Serious confusion as to the structure of butaprost was generated by Gardiner in 1986,{3326} when he reported that the epimer of butaprost showing this selective activity was the C-16 (R)-epimer (See reference 2 and NOTE). In order to increase the binding affinity of (R)-butaprost for prostanoid receptors, we removed the methyl ester of (R)-butaprost and re-established the natural C-1 carboxylic acid. Prostaglandin free acids generally bind to their cognate receptors with 10 to 100 times the affinity of the corresponding ester derivative. The pharmacology of (R)-butaprost has not been carefully studied, but it is generally considered to be the less active C-16 epimer.{3178} (NOTE: In the Gardiner paper in the 1986 British Journal of Pharmacology, butaprost appears on page 46 where it is given the name TR 4979. The structure as drawn is incorrect, in that the author was using and referring to the more active C-16 epimer, which is actually 16(S). The structure on page 46 shows the structure as 16(R). It was not until the late 1990’s that careful studies both in the US and Japan correctly identified the actual configuration of C-16 in the compound called butaprost is 16(S).){3326}
Brand:CaymanSKU:10006045 - 1 mgAvailable on backorder
Butaprost is a structural analog of prostaglandin E2 (PGE2) with good selectivity for the EP2 receptor subtype. Butaprost has frequently been used to pharmacologically define the EP receptor expression profile of various human and animal tissues and cells.{3317} Serious confusion as to the structure of butaprost was generated by Gardiner in 1986,{3326} when he reported that the epimer of butaprost showing this selective activity was the C-16 (R)-epimer (See reference 2 and NOTE). In order to increase the binding affinity of (R)-butaprost for prostanoid receptors, we removed the methyl ester of (R)-butaprost and re-established the natural C-1 carboxylic acid. Prostaglandin free acids generally bind to their cognate receptors with 10 to 100 times the affinity of the corresponding ester derivative. The pharmacology of (R)-butaprost has not been carefully studied, but it is generally considered to be the less active C-16 epimer.{3178} (NOTE: In the Gardiner paper in the 1986 British Journal of Pharmacology, butaprost appears on page 46 where it is given the name TR 4979. The structure as drawn is incorrect, in that the author was using and referring to the more active C-16 epimer, which is actually 16(S). The structure on page 46 shows the structure as 16(R). It was not until the late 1990’s that careful studies both in the US and Japan correctly identified the actual configuration of C-16 in the compound called butaprost is 16(S).){3326}
Brand:CaymanSKU:10006045 - 10 mgAvailable on backorder
Butaprost is a structural analog of prostaglandin E2 (PGE2) with good selectivity for the EP2 receptor subtype. Butaprost has frequently been used to pharmacologically define the EP receptor expression profile of various human and animal tissues and cells.{3317} Serious confusion as to the structure of butaprost was generated by Gardiner in 1986,{3326} when he reported that the epimer of butaprost showing this selective activity was the C-16 (R)-epimer (See reference 2 and NOTE). In order to increase the binding affinity of (R)-butaprost for prostanoid receptors, we removed the methyl ester of (R)-butaprost and re-established the natural C-1 carboxylic acid. Prostaglandin free acids generally bind to their cognate receptors with 10 to 100 times the affinity of the corresponding ester derivative. The pharmacology of (R)-butaprost has not been carefully studied, but it is generally considered to be the less active C-16 epimer.{3178} (NOTE: In the Gardiner paper in the 1986 British Journal of Pharmacology, butaprost appears on page 46 where it is given the name TR 4979. The structure as drawn is incorrect, in that the author was using and referring to the more active C-16 epimer, which is actually 16(S). The structure on page 46 shows the structure as 16(R). It was not until the late 1990’s that careful studies both in the US and Japan correctly identified the actual configuration of C-16 in the compound called butaprost is 16(S).){3326}
Brand:CaymanSKU:10006045 - 5 mgAvailable on backorder
Butaprost is a structural analog of prostaglandin E2 (PGE2) with good selectivity for the EP2 receptor subtype. Butaprost has frequently been used to pharmacologically define the EP receptor expression profile of various human and animal tissues and cells.{3317} Serious confusion as to the structure of butaprost was generated by Gardiner in 1986,{3326} when he reported that the epimer of butaprost showing this selective activity was the C-16 (R)-epimer (See reference 2 and NOTE). In order to increase the binding affinity of (R)-butaprost for prostanoid receptors, we removed the methyl ester of (R)-butaprost and re-established the natural C-1 carboxylic acid. Prostaglandin free acids generally bind to their cognate receptors with 10 to 100 times the affinity of the corresponding ester derivative. The pharmacology of (R)-butaprost has not been carefully studied, but it is generally considered to be the less active C-16 epimer.{3178} (NOTE: In the Gardiner paper in the 1986 British Journal of Pharmacology, butaprost appears on page 46 where it is given the name TR 4979. The structure as drawn is incorrect, in that the author was using and referring to the more active C-16 epimer, which is actually 16(S). The structure on page 46 shows the structure as 16(R). It was not until the late 1990’s that careful studies both in the US and Japan correctly identified the actual configuration of C-16 in the compound called butaprost is 16(S).){3326}
Brand:CaymanSKU:10006045 - 500 µgAvailable on backorder
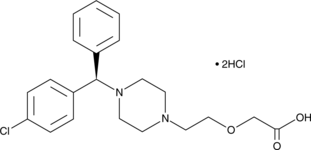
(R)-Cetirizine is the (R)-enantiomer of the histamine H1 receptor antagonist and second generation antihistamine cetirizine (Item No. 19686).{31604} (R)-Cetirizine binds to the H1 receptor with higher affinity than cetirizine (Kis = 3 and 6 nM, respectively) and is 25,000-100,000-fold selective for H1 receptors over muscarinic M1-M5 receptors.{39482} It decreases production of RANTES and eotaxin following antigen stimulation in mouse eosinophils in vitro in a concentration-dependent manner with a minimum effective concentration (MEC) of 0.05 µM.{39483} Intranasal administration of (R)-cetirizine (0.01-1%) dose-dependently decreases histamine-induced nasal rubbing and sneezing in mice.{39484} Formulations containing (R)-cetirizine have been used in the treatment of allergic rhinitis and chronic idiopathic urticaria.
Brand:CaymanSKU:23992 - 10 mgAvailable on backorder
(R)-Cetirizine is the (R)-enantiomer of the histamine H1 receptor antagonist and second generation antihistamine cetirizine (Item No. 19686).{31604} (R)-Cetirizine binds to the H1 receptor with higher affinity than cetirizine (Kis = 3 and 6 nM, respectively) and is 25,000-100,000-fold selective for H1 receptors over muscarinic M1-M5 receptors.{39482} It decreases production of RANTES and eotaxin following antigen stimulation in mouse eosinophils in vitro in a concentration-dependent manner with a minimum effective concentration (MEC) of 0.05 µM.{39483} Intranasal administration of (R)-cetirizine (0.01-1%) dose-dependently decreases histamine-induced nasal rubbing and sneezing in mice.{39484} Formulations containing (R)-cetirizine have been used in the treatment of allergic rhinitis and chronic idiopathic urticaria.
Brand:CaymanSKU:23992 - 100 mgAvailable on backorder
(R)-Cetirizine is the (R)-enantiomer of the histamine H1 receptor antagonist and second generation antihistamine cetirizine (Item No. 19686).{31604} (R)-Cetirizine binds to the H1 receptor with higher affinity than cetirizine (Kis = 3 and 6 nM, respectively) and is 25,000-100,000-fold selective for H1 receptors over muscarinic M1-M5 receptors.{39482} It decreases production of RANTES and eotaxin following antigen stimulation in mouse eosinophils in vitro in a concentration-dependent manner with a minimum effective concentration (MEC) of 0.05 µM.{39483} Intranasal administration of (R)-cetirizine (0.01-1%) dose-dependently decreases histamine-induced nasal rubbing and sneezing in mice.{39484} Formulations containing (R)-cetirizine have been used in the treatment of allergic rhinitis and chronic idiopathic urticaria.
Brand:CaymanSKU:23992 - 250 mgAvailable on backorder
(R)-Cetirizine is the (R)-enantiomer of the histamine H1 receptor antagonist and second generation antihistamine cetirizine (Item No. 19686).{31604} (R)-Cetirizine binds to the H1 receptor with higher affinity than cetirizine (Kis = 3 and 6 nM, respectively) and is 25,000-100,000-fold selective for H1 receptors over muscarinic M1-M5 receptors.{39482} It decreases production of RANTES and eotaxin following antigen stimulation in mouse eosinophils in vitro in a concentration-dependent manner with a minimum effective concentration (MEC) of 0.05 µM.{39483} Intranasal administration of (R)-cetirizine (0.01-1%) dose-dependently decreases histamine-induced nasal rubbing and sneezing in mice.{39484} Formulations containing (R)-cetirizine have been used in the treatment of allergic rhinitis and chronic idiopathic urticaria.
Brand:CaymanSKU:23992 - 50 mgAvailable on backorder
(R)-CPP is an NMDA receptor antagonist (Ki = 0.14 µM).{51092} It binds to NMDA receptors containing GluN2A, GluN2B, GluN2C, and GluN2D subunits with Ki values of 0.04, 0.3, 0.6, and 2 µM, respectively.{51093} It inhibits depolarization induced by NMDA in isolated hemisected frog spinal cord (pA2 = 6.56) and NMDA-induced sodium efflux from rat brain slices (pA2 = 6.2).{51092} (R)-CPP inhibits the clonic phase of sound-induced seizures in DBA/2 mice (ED50 = 65.8 µmol/kg) and the myoclonic phase of stroboscopic-induced seizures in P. papio photosensitive baboons (ED50 = 127 µmol/kg).{51094}
Brand:CaymanSKU:21569 -Out of stock
(R)-CPP is an NMDA receptor antagonist (Ki = 0.14 µM).{51092} It binds to NMDA receptors containing GluN2A, GluN2B, GluN2C, and GluN2D subunits with Ki values of 0.04, 0.3, 0.6, and 2 µM, respectively.{51093} It inhibits depolarization induced by NMDA in isolated hemisected frog spinal cord (pA2 = 6.56) and NMDA-induced sodium efflux from rat brain slices (pA2 = 6.2).{51092} (R)-CPP inhibits the clonic phase of sound-induced seizures in DBA/2 mice (ED50 = 65.8 µmol/kg) and the myoclonic phase of stroboscopic-induced seizures in P. papio photosensitive baboons (ED50 = 127 µmol/kg).{51094}
Brand:CaymanSKU:21569 -Out of stock
(R)-CPP is an NMDA receptor antagonist (Ki = 0.14 µM).{51092} It binds to NMDA receptors containing GluN2A, GluN2B, GluN2C, and GluN2D subunits with Ki values of 0.04, 0.3, 0.6, and 2 µM, respectively.{51093} It inhibits depolarization induced by NMDA in isolated hemisected frog spinal cord (pA2 = 6.56) and NMDA-induced sodium efflux from rat brain slices (pA2 = 6.2).{51092} (R)-CPP inhibits the clonic phase of sound-induced seizures in DBA/2 mice (ED50 = 65.8 µmol/kg) and the myoclonic phase of stroboscopic-induced seizures in P. papio photosensitive baboons (ED50 = 127 µmol/kg).{51094}
Brand:CaymanSKU:21569 -Out of stock
(R)-CPP is an NMDA receptor antagonist (Ki = 0.14 µM).{51092} It binds to NMDA receptors containing GluN2A, GluN2B, GluN2C, and GluN2D subunits with Ki values of 0.04, 0.3, 0.6, and 2 µM, respectively.{51093} It inhibits depolarization induced by NMDA in isolated hemisected frog spinal cord (pA2 = 6.56) and NMDA-induced sodium efflux from rat brain slices (pA2 = 6.2).{51092} (R)-CPP inhibits the clonic phase of sound-induced seizures in DBA/2 mice (ED50 = 65.8 µmol/kg) and the myoclonic phase of stroboscopic-induced seizures in P. papio photosensitive baboons (ED50 = 127 µmol/kg).{51094}
Brand:CaymanSKU:21569 -Out of stock
Cyclin-dependent kinases (CDKs) are key regulators of cell cycle progression and are therefore promising targets for cancer therapy.{21810} (R)-CR8 is a second-generation analog of (R)-roscovitine (Item No. 10009569) that inhibits Cdk1/cyclin B, Cdk2/cyclin A, Cdk2/cyclin E, Cdk5/p25, and Cdk9/cyclin T with IC50 values of 0.09, 0.072, 0.041, 0.11, and 0.18 μM, respectively.{21811,21809} (R)-CR8 has 2- to 4-fold improved potency for the inhibition of CDKs over (R)-roscovitine and can inhibit the proliferation of various cancer cell lines with ~40-fold more potency than (R)-roscovitine (IC50s ~ 0.39 versus 27.8 μM, respectively).{21811,21809} (R)-CR8 also inhibits casein kinase 1 (CK1δ/ε) with an IC50 value of 0.40 μM and inhibits GSK3α/β with an IC50 value of 12 μM.{21811}
Brand:CaymanSKU:-Cyclin-dependent kinases (CDKs) are key regulators of cell cycle progression and are therefore promising targets for cancer therapy.{21810} (R)-CR8 is a second-generation analog of (R)-roscovitine (Item No. 10009569) that inhibits Cdk1/cyclin B, Cdk2/cyclin A, Cdk2/cyclin E, Cdk5/p25, and Cdk9/cyclin T with IC50 values of 0.09, 0.072, 0.041, 0.11, and 0.18 μM, respectively.{21811,21809} (R)-CR8 has 2- to 4-fold improved potency for the inhibition of CDKs over (R)-roscovitine and can inhibit the proliferation of various cancer cell lines with ~40-fold more potency than (R)-roscovitine (IC50s ~ 0.39 versus 27.8 μM, respectively).{21811,21809} (R)-CR8 also inhibits casein kinase 1 (CK1δ/ε) with an IC50 value of 0.40 μM and inhibits GSK3α/β with an IC50 value of 12 μM.{21811}
Brand:CaymanSKU:-Cyclin-dependent kinases (CDKs) are key regulators of cell cycle progression and are therefore promising targets for cancer therapy.{21810} (R)-CR8 is a second-generation analog of (R)-roscovitine (Item No. 10009569) that inhibits Cdk1/cyclin B, Cdk2/cyclin A, Cdk2/cyclin E, Cdk5/p25, and Cdk9/cyclin T with IC50 values of 0.09, 0.072, 0.041, 0.11, and 0.18 μM, respectively.{21811,21809} (R)-CR8 has 2- to 4-fold improved potency for the inhibition of CDKs over (R)-roscovitine and can inhibit the proliferation of various cancer cell lines with ~40-fold more potency than (R)-roscovitine (IC50s ~ 0.39 versus 27.8 μM, respectively).{21811,21809} (R)-CR8 also inhibits casein kinase 1 (CK1δ/ε) with an IC50 value of 0.40 μM and inhibits GSK3α/β with an IC50 value of 12 μM.{21811}
Brand:CaymanSKU:-(R)-Crizotinib is a derivative of aminopyridine that acts as a potent, orally bioavailable, ATP-competitive small-molecule dual inhibitor of c-MET (IC50 = 8 nM) and ALK (IC50 = 20 nM) receptor tyrosine kinases.{22302} (R)-Crizotinib shows antitumor efficacy, including cytoreductive antitumor activity, in multiple tumor models implanted in athymic mice that express activated c-MET or ALK fusion proteins (IC50s = 5-20 nM).{22302,22301} (R)-Crizotinib is currently undergoing active clinical investigation in non-small cell lung cancer and phase I/II studies are being conducted in patients with anaplastic large cell lymphoma or neuroblastoma.{22301,20554}
Brand:CaymanSKU:12087 - 10 mgAvailable on backorder
(R)-Crizotinib is a derivative of aminopyridine that acts as a potent, orally bioavailable, ATP-competitive small-molecule dual inhibitor of c-MET (IC50 = 8 nM) and ALK (IC50 = 20 nM) receptor tyrosine kinases.{22302} (R)-Crizotinib shows antitumor efficacy, including cytoreductive antitumor activity, in multiple tumor models implanted in athymic mice that express activated c-MET or ALK fusion proteins (IC50s = 5-20 nM).{22302,22301} (R)-Crizotinib is currently undergoing active clinical investigation in non-small cell lung cancer and phase I/II studies are being conducted in patients with anaplastic large cell lymphoma or neuroblastoma.{22301,20554}
Brand:CaymanSKU:12087 - 5 mgAvailable on backorder
(R)-Crizotinib is a derivative of aminopyridine that acts as a potent, orally bioavailable, ATP-competitive small-molecule dual inhibitor of c-MET (IC50 = 8 nM) and ALK (IC50 = 20 nM) receptor tyrosine kinases.{22302} (R)-Crizotinib shows antitumor efficacy, including cytoreductive antitumor activity, in multiple tumor models implanted in athymic mice that express activated c-MET or ALK fusion proteins (IC50s = 5-20 nM).{22302,22301} (R)-Crizotinib is currently undergoing active clinical investigation in non-small cell lung cancer and phase I/II studies are being conducted in patients with anaplastic large cell lymphoma or neuroblastoma.{22301,20554}
Brand:CaymanSKU:12087 - 50 mgAvailable on backorder
(R)-Crizotinib-d5 is intended for use as an internal standard for the quantification of (R)-crizotinib (Item No. 12087) by GC- or LC-MS. (R)-Crizotinib is a derivative of aminopyridine that acts as a potent, orally bioavailable, ATP-competitive small-molecule dual inhibitor of c-MET (IC50 = 8 nM) and ALK (IC50 = 20 nM) receptor tyrosine kinases.{22302} (R)-Crizotinib shows antitumor efficacy, including cytoreductive antitumor activity, in multiple tumor models implanted in athymic mice that express activated c-MET or ALK fusion proteins (IC50s = 5-20 nM).{22302,22301}
Brand:CaymanSKU:26762 - 1 mgAvailable on backorder
(R)-Crizotinib-d5 is intended for use as an internal standard for the quantification of (R)-crizotinib (Item No. 12087) by GC- or LC-MS. (R)-Crizotinib is a derivative of aminopyridine that acts as a potent, orally bioavailable, ATP-competitive small-molecule dual inhibitor of c-MET (IC50 = 8 nM) and ALK (IC50 = 20 nM) receptor tyrosine kinases.{22302} (R)-Crizotinib shows antitumor efficacy, including cytoreductive antitumor activity, in multiple tumor models implanted in athymic mice that express activated c-MET or ALK fusion proteins (IC50s = 5-20 nM).{22302,22301}
Brand:CaymanSKU:26762 - 500 µgAvailable on backorder
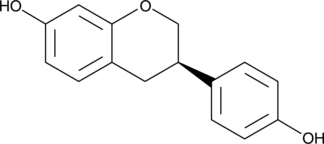
Equol is a non-steroidal estrogen produced from the metabolism of the isoflavonoid phytoestrogen daidzein by human intestinal microflora.{16628,16720} It is a chiral molecule that exists in two enantiomeric forms.{16720} In contrast to the estrogen receptor (ER) selectivity of (S)-equol , (R)-equol is a weaker ER agonist that binds to ERα and ERβ with Ki values of 27.4 and 15.4 nM, respectively.{16628} The (R)-enantiomer demonstrates higher ER agonist activity at ERα compared to ERβ (EC50 = 66 and 330 nM, respectively).{16720}
Brand:CaymanSKU:10010172 - 1 mgAvailable on backorder
Equol is a non-steroidal estrogen produced from the metabolism of the isoflavonoid phytoestrogen daidzein by human intestinal microflora.{16628,16720} It is a chiral molecule that exists in two enantiomeric forms.{16720} In contrast to the estrogen receptor (ER) selectivity of (S)-equol , (R)-equol is a weaker ER agonist that binds to ERα and ERβ with Ki values of 27.4 and 15.4 nM, respectively.{16628} The (R)-enantiomer demonstrates higher ER agonist activity at ERα compared to ERβ (EC50 = 66 and 330 nM, respectively).{16720}
Brand:CaymanSKU:10010172 - 10 mgAvailable on backorder
Equol is a non-steroidal estrogen produced from the metabolism of the isoflavonoid phytoestrogen daidzein by human intestinal microflora.{16628,16720} It is a chiral molecule that exists in two enantiomeric forms.{16720} In contrast to the estrogen receptor (ER) selectivity of (S)-equol , (R)-equol is a weaker ER agonist that binds to ERα and ERβ with Ki values of 27.4 and 15.4 nM, respectively.{16628} The (R)-enantiomer demonstrates higher ER agonist activity at ERα compared to ERβ (EC50 = 66 and 330 nM, respectively).{16720}
Brand:CaymanSKU:10010172 - 25 mgAvailable on backorder
Equol is a non-steroidal estrogen produced from the metabolism of the isoflavonoid phytoestrogen daidzein by human intestinal microflora.{16628,16720} It is a chiral molecule that exists in two enantiomeric forms.{16720} In contrast to the estrogen receptor (ER) selectivity of (S)-equol , (R)-equol is a weaker ER agonist that binds to ERα and ERβ with Ki values of 27.4 and 15.4 nM, respectively.{16628} The (R)-enantiomer demonstrates higher ER agonist activity at ERα compared to ERβ (EC50 = 66 and 330 nM, respectively).{16720}
Brand:CaymanSKU:10010172 - 5 mgAvailable on backorder
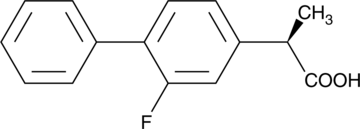
(R)-Flurbiprofen is a COX-inactive enantiomer of the racemic non-selective COX inhibitor flurbiprofen (Item No. 70250) that has diverse biological activities.{16203,16204,16205,39781} It inhibits γ-secretase activity in vitro and, in vivo, it reduces formation of amyloid-β peptide 1-42 (Aβ42) and improves axonal transport in young Aβ-plaque free mice but not old mice with existing Aβ plaques in the Tg2576 transgenic model of Alzheimer’s disease.{16203,39781} (R)-Flurbiprofen inhibits NF-kB activation and DNA binding as well as AP-1 DNA binding in RAW 264.7 macrophages and reduces paw edema in a rat model of zymosan-induced inflammation via COX-independent inhibition of NF-κB and AP-1 activation when administered at doses of 1, 3, and 9 mg/kg.{16204} It also suppresses prostate tumor cell growth in vitro by inducing p75NTR protein expression and reduces tumor growth and metastasis in multiple mouse models of intestinal neoplasia.{16205,16204}
Brand:CaymanSKU:70255 - 1 gAvailable on backorder
(R)-Flurbiprofen is a COX-inactive enantiomer of the racemic non-selective COX inhibitor flurbiprofen (Item No. 70250) that has diverse biological activities.{16203,16204,16205,39781} It inhibits γ-secretase activity in vitro and, in vivo, it reduces formation of amyloid-β peptide 1-42 (Aβ42) and improves axonal transport in young Aβ-plaque free mice but not old mice with existing Aβ plaques in the Tg2576 transgenic model of Alzheimer’s disease.{16203,39781} (R)-Flurbiprofen inhibits NF-kB activation and DNA binding as well as AP-1 DNA binding in RAW 264.7 macrophages and reduces paw edema in a rat model of zymosan-induced inflammation via COX-independent inhibition of NF-κB and AP-1 activation when administered at doses of 1, 3, and 9 mg/kg.{16204} It also suppresses prostate tumor cell growth in vitro by inducing p75NTR protein expression and reduces tumor growth and metastasis in multiple mouse models of intestinal neoplasia.{16205,16204}
Brand:CaymanSKU:70255 - 100 mgAvailable on backorder
(R)-Flurbiprofen is a COX-inactive enantiomer of the racemic non-selective COX inhibitor flurbiprofen (Item No. 70250) that has diverse biological activities.{16203,16204,16205,39781} It inhibits γ-secretase activity in vitro and, in vivo, it reduces formation of amyloid-β peptide 1-42 (Aβ42) and improves axonal transport in young Aβ-plaque free mice but not old mice with existing Aβ plaques in the Tg2576 transgenic model of Alzheimer’s disease.{16203,39781} (R)-Flurbiprofen inhibits NF-kB activation and DNA binding as well as AP-1 DNA binding in RAW 264.7 macrophages and reduces paw edema in a rat model of zymosan-induced inflammation via COX-independent inhibition of NF-κB and AP-1 activation when administered at doses of 1, 3, and 9 mg/kg.{16204} It also suppresses prostate tumor cell growth in vitro by inducing p75NTR protein expression and reduces tumor growth and metastasis in multiple mouse models of intestinal neoplasia.{16205,16204}
Brand:CaymanSKU:70255 - 250 mgAvailable on backorder
(R)-Flurbiprofen is a COX-inactive enantiomer of the racemic non-selective COX inhibitor flurbiprofen (Item No. 70250) that has diverse biological activities.{16203,16204,16205,39781} It inhibits γ-secretase activity in vitro and, in vivo, it reduces formation of amyloid-β peptide 1-42 (Aβ42) and improves axonal transport in young Aβ-plaque free mice but not old mice with existing Aβ plaques in the Tg2576 transgenic model of Alzheimer’s disease.{16203,39781} (R)-Flurbiprofen inhibits NF-kB activation and DNA binding as well as AP-1 DNA binding in RAW 264.7 macrophages and reduces paw edema in a rat model of zymosan-induced inflammation via COX-independent inhibition of NF-κB and AP-1 activation when administered at doses of 1, 3, and 9 mg/kg.{16204} It also suppresses prostate tumor cell growth in vitro by inducing p75NTR protein expression and reduces tumor growth and metastasis in multiple mouse models of intestinal neoplasia.{16205,16204}
Brand:CaymanSKU:70255 - 500 mgAvailable on backorder
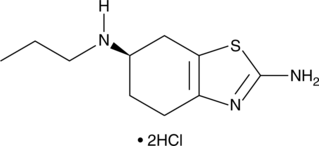
(R)-Gyramide A is a bacterial DNA gyrase inhibitor that disrupts supercoiling activity with an IC50 value of 3.3 µM.{29792} It does not affect the closely related enzyme topoisomerase IV.{29793} (R)-Gyramide A demonstrates antibacterial activity against E. coli, P. aeruginosa, and S. enterica (MICs range from 10-80 µM).{29792}
Brand:CaymanSKU:-Available on backorder
(R)-Gyramide A is a bacterial DNA gyrase inhibitor that disrupts supercoiling activity with an IC50 value of 3.3 µM.{29792} It does not affect the closely related enzyme topoisomerase IV.{29793} (R)-Gyramide A demonstrates antibacterial activity against E. coli, P. aeruginosa, and S. enterica (MICs range from 10-80 µM).{29792}
Brand:CaymanSKU:-Available on backorder
(R)-Gyramide A is a bacterial DNA gyrase inhibitor that disrupts supercoiling activity with an IC50 value of 3.3 µM.{29792} It does not affect the closely related enzyme topoisomerase IV.{29793} (R)-Gyramide A demonstrates antibacterial activity against E. coli, P. aeruginosa, and S. enterica (MICs range from 10-80 µM).{29792}
Brand:CaymanSKU:-Available on backorder
(R)-Gyramide A is a bacterial DNA gyrase inhibitor that disrupts supercoiling activity with an IC50 value of 3.3 µM.{29792} It does not affect the closely related enzyme topoisomerase IV.{29793} (R)-Gyramide A demonstrates antibacterial activity against E. coli, P. aeruginosa, and S. enterica (MICs range from 10-80 µM).{29792}
Brand:CaymanSKU:-Available on backorder
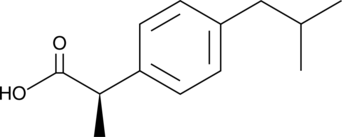
Ibuprofen is a non-steroidal anti-inflammatory drug with diverse biochemical actions, most notably inhibiting COX-1 and COX-2 (IC50s = 2.6 and 1.53 µM, respectively).{1286} It is commonly synthesized as a racemic mixture of (S) and (R) isomers (Item No. item 70280). (R)-Ibuprofen is an enantiomer that is generally not considered a COX inhibitor and is instead thought to be involved in pathways of lipid metabolism as it is incorporated into triglycerides along with fatty acids.{27416} The (R) enantiomer can, however, inhibit NF-κB activation (IC50 = 121.8 µM) in response to T-cell stimulation as well as block superoxide formation, β-glucuronidase release, and LTB4 generation by stimulated neutrophils (IC50 values range from 40-100 µM).{6935,27418} 50-60% of (R)-ibuprofen is inverted to (S)-ibuprofen (Item No. 16793) in humans after oral administration.{27416,27417,10032}
Brand:CaymanSKU:-Out of stock
Ibuprofen is a non-steroidal anti-inflammatory drug with diverse biochemical actions, most notably inhibiting COX-1 and COX-2 (IC50s = 2.6 and 1.53 µM, respectively).{1286} It is commonly synthesized as a racemic mixture of (S) and (R) isomers (Item No. item 70280). (R)-Ibuprofen is an enantiomer that is generally not considered a COX inhibitor and is instead thought to be involved in pathways of lipid metabolism as it is incorporated into triglycerides along with fatty acids.{27416} The (R) enantiomer can, however, inhibit NF-κB activation (IC50 = 121.8 µM) in response to T-cell stimulation as well as block superoxide formation, β-glucuronidase release, and LTB4 generation by stimulated neutrophils (IC50 values range from 40-100 µM).{6935,27418} 50-60% of (R)-ibuprofen is inverted to (S)-ibuprofen (Item No. 16793) in humans after oral administration.{27416,27417,10032}
Brand:CaymanSKU:-Out of stock
Ibuprofen is a non-steroidal anti-inflammatory drug with diverse biochemical actions, most notably inhibiting COX-1 and COX-2 (IC50s = 2.6 and 1.53 µM, respectively).{1286} It is commonly synthesized as a racemic mixture of (S) and (R) isomers (Item No. item 70280). (R)-Ibuprofen is an enantiomer that is generally not considered a COX inhibitor and is instead thought to be involved in pathways of lipid metabolism as it is incorporated into triglycerides along with fatty acids.{27416} The (R) enantiomer can, however, inhibit NF-κB activation (IC50 = 121.8 µM) in response to T-cell stimulation as well as block superoxide formation, β-glucuronidase release, and LTB4 generation by stimulated neutrophils (IC50 values range from 40-100 µM).{6935,27418} 50-60% of (R)-ibuprofen is inverted to (S)-ibuprofen (Item No. 16793) in humans after oral administration.{27416,27417,10032}
Brand:CaymanSKU:-Out of stock
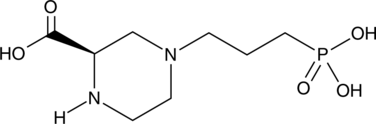
(R)-KT109 is the (R) isomer of the diacylglycerol lipase β (DAGLβ) inhibitor KT109 (Item No. 18933).{43435} (R)-KT109 is an inhibitor of DAGLβ (IC50 = 0.79 nM) and of DAGLα-mediated hydrolysis of 1-stearoyl-2-arachidonoyl-sn-glycerol (Item No. 10008650; IC50 = 25.12 nM). It also inhibits α/β-hydrolase domain-containing protein 6 (ABHD6) with an IC50 value of 2.51 nM. (R)-KT109 is more potent at DAGLβ, DAGLα, and ABHD6 than (S)-KT109 (Item No. 25683).
Brand:CaymanSKU:25682 - 1 mgAvailable on backorder
(R)-KT109 is the (R) isomer of the diacylglycerol lipase β (DAGLβ) inhibitor KT109 (Item No. 18933).{43435} (R)-KT109 is an inhibitor of DAGLβ (IC50 = 0.79 nM) and of DAGLα-mediated hydrolysis of 1-stearoyl-2-arachidonoyl-sn-glycerol (Item No. 10008650; IC50 = 25.12 nM). It also inhibits α/β-hydrolase domain-containing protein 6 (ABHD6) with an IC50 value of 2.51 nM. (R)-KT109 is more potent at DAGLβ, DAGLα, and ABHD6 than (S)-KT109 (Item No. 25683).
Brand:CaymanSKU:25682 - 500 µgAvailable on backorder
Lansoprazole (Item No. 13627) is a proton pump inhibitor that irreversibly inactivates the H+/K+-stimulated ATPase pumps in parietal cells, inhibiting gastric acid secretion and increasing intragastric pH.{18250} It is a 1:1 racemic mixture of (R)-lansoprazole and (S)-lansoprazole, both of which are pharmacologically active.{29367} (R)-Lansoprazole is an enantiomerically pure form of lansoprazole. It can inhibit acid formation in isolated canine parietal cells with an IC50 value of 59 nM and inhibit the H+/K+-ATPase with an IC50 value of 4.2 µM.{29367}
Brand:CaymanSKU:-Available on backorder
Lansoprazole (Item No. 13627) is a proton pump inhibitor that irreversibly inactivates the H+/K+-stimulated ATPase pumps in parietal cells, inhibiting gastric acid secretion and increasing intragastric pH.{18250} It is a 1:1 racemic mixture of (R)-lansoprazole and (S)-lansoprazole, both of which are pharmacologically active.{29367} (R)-Lansoprazole is an enantiomerically pure form of lansoprazole. It can inhibit acid formation in isolated canine parietal cells with an IC50 value of 59 nM and inhibit the H+/K+-ATPase with an IC50 value of 4.2 µM.{29367}
Brand:CaymanSKU:-Available on backorder
Lansoprazole (Item No. 13627) is a proton pump inhibitor that irreversibly inactivates the H+/K+-stimulated ATPase pumps in parietal cells, inhibiting gastric acid secretion and increasing intragastric pH.{18250} It is a 1:1 racemic mixture of (R)-lansoprazole and (S)-lansoprazole, both of which are pharmacologically active.{29367} (R)-Lansoprazole is an enantiomerically pure form of lansoprazole. It can inhibit acid formation in isolated canine parietal cells with an IC50 value of 59 nM and inhibit the H+/K+-ATPase with an IC50 value of 4.2 µM.{29367}
Brand:CaymanSKU:-Available on backorder
LSF, a chiral metabolite of pentoxifylline, acts as a potent anti-inflammatory agent.{17416,18148} (R)-LSF is the biologically active isomer of LSF.{17416,18148} It is a potent inhibitor of the generation of phosphatidic acid (IC50 = 0.6 µM) from cytokine-activated lysophosphatidic acyl transferase (LPAAT), which has been shown to protect mice from endotoxic shock.{17415} (R)-LSF suppresses the production of the proinflammatory cytokine IFN-γ, inhibits interleukin 12-mediated STAT-4 activation, and enhances glucose-stimulated β-cell insulin secretion, reducing the onset of diabetes in a non-obese diabetic mouse model.{17414,17416}
Brand:CaymanSKU:-LSF, a chiral metabolite of pentoxifylline, acts as a potent anti-inflammatory agent.{17416,18148} (R)-LSF is the biologically active isomer of LSF.{17416,18148} It is a potent inhibitor of the generation of phosphatidic acid (IC50 = 0.6 µM) from cytokine-activated lysophosphatidic acyl transferase (LPAAT), which has been shown to protect mice from endotoxic shock.{17415} (R)-LSF suppresses the production of the proinflammatory cytokine IFN-γ, inhibits interleukin 12-mediated STAT-4 activation, and enhances glucose-stimulated β-cell insulin secretion, reducing the onset of diabetes in a non-obese diabetic mouse model.{17414,17416}
Brand:CaymanSKU:-LSF, a chiral metabolite of pentoxifylline, acts as a potent anti-inflammatory agent.{17416,18148} (R)-LSF is the biologically active isomer of LSF.{17416,18148} It is a potent inhibitor of the generation of phosphatidic acid (IC50 = 0.6 µM) from cytokine-activated lysophosphatidic acyl transferase (LPAAT), which has been shown to protect mice from endotoxic shock.{17415} (R)-LSF suppresses the production of the proinflammatory cytokine IFN-γ, inhibits interleukin 12-mediated STAT-4 activation, and enhances glucose-stimulated β-cell insulin secretion, reducing the onset of diabetes in a non-obese diabetic mouse model.{17414,17416}
Brand:CaymanSKU:-(R)-Mephenytoin is the (R) enantiomer of the anticonvulsant mephenytoin.{47078} (R)-Mephenytoin can be demethylated by the cytochrome P450 (CYP) isoform CYP2C9 to form the active metabolite 5-ethyl-5-phenylhydantoin (nirvanol).{47079,23860} The ratio of (S)-mephenytoin (Item No. 11913) to (R)-mephenytoin in urine following administration of the racemic mixture has been used to detect polymorphisms in drug metabolism by CYP2C19, as only (S)-mephenytoin is a substrate of CYP2C19.{23861,23857,47079}
Brand:CaymanSKU:25891 - 10 mgAvailable on backorder
(R)-Mephenytoin is the (R) enantiomer of the anticonvulsant mephenytoin.{47078} (R)-Mephenytoin can be demethylated by the cytochrome P450 (CYP) isoform CYP2C9 to form the active metabolite 5-ethyl-5-phenylhydantoin (nirvanol).{47079,23860} The ratio of (S)-mephenytoin (Item No. 11913) to (R)-mephenytoin in urine following administration of the racemic mixture has been used to detect polymorphisms in drug metabolism by CYP2C19, as only (S)-mephenytoin is a substrate of CYP2C19.{23861,23857,47079}
Brand:CaymanSKU:25891 - 100 mgAvailable on backorder
(R)-Mephenytoin is the (R) enantiomer of the anticonvulsant mephenytoin.{47078} (R)-Mephenytoin can be demethylated by the cytochrome P450 (CYP) isoform CYP2C9 to form the active metabolite 5-ethyl-5-phenylhydantoin (nirvanol).{47079,23860} The ratio of (S)-mephenytoin (Item No. 11913) to (R)-mephenytoin in urine following administration of the racemic mixture has been used to detect polymorphisms in drug metabolism by CYP2C19, as only (S)-mephenytoin is a substrate of CYP2C19.{23861,23857,47079}
Brand:CaymanSKU:25891 - 25 mgAvailable on backorder
(R)-Mephenytoin is the (R) enantiomer of the anticonvulsant mephenytoin.{47078} (R)-Mephenytoin can be demethylated by the cytochrome P450 (CYP) isoform CYP2C9 to form the active metabolite 5-ethyl-5-phenylhydantoin (nirvanol).{47079,23860} The ratio of (S)-mephenytoin (Item No. 11913) to (R)-mephenytoin in urine following administration of the racemic mixture has been used to detect polymorphisms in drug metabolism by CYP2C19, as only (S)-mephenytoin is a substrate of CYP2C19.{23861,23857,47079}
Brand:CaymanSKU:25891 - 50 mgAvailable on backorder
The ubiquitin-proteasome pathway plays an integral role in the selective degradation of intracellular proteins. While important for clearing damaged or mis-folded proteins, this proteolytic pathway also regulates the availability of key proteins involved in the control of inflammatory processes, cell cycle regulation, and gene expression.{13818,13345} (R)-MG132 is a potent, reversible, and cell permeable proteasome inhibitor. After treatment for one hour at 100 nM, it inhibits 50% and 31% of proteasome activity in lysates of J558L multiple myeloma cells and EMT6 breast cancer cells, respectively.{17933} The (R)-MG132 stereoisomer is a more effective inhibitor of chymotrypsin-like (ChTL), trypsin-like (TL), and peptidylglutamyl peptide hydrolyzing proteasome (PGPH) activities compared to (S)-MG132 (IC50s = 0.22 versus 0.89 µM (ChTL); 34.4 versus 104.43 µM (TL); 2.95 versus 5.70 µM (PGPH), respectively).{17933}
Brand:CaymanSKU:-The ubiquitin-proteasome pathway plays an integral role in the selective degradation of intracellular proteins. While important for clearing damaged or mis-folded proteins, this proteolytic pathway also regulates the availability of key proteins involved in the control of inflammatory processes, cell cycle regulation, and gene expression.{13818,13345} (R)-MG132 is a potent, reversible, and cell permeable proteasome inhibitor. After treatment for one hour at 100 nM, it inhibits 50% and 31% of proteasome activity in lysates of J558L multiple myeloma cells and EMT6 breast cancer cells, respectively.{17933} The (R)-MG132 stereoisomer is a more effective inhibitor of chymotrypsin-like (ChTL), trypsin-like (TL), and peptidylglutamyl peptide hydrolyzing proteasome (PGPH) activities compared to (S)-MG132 (IC50s = 0.22 versus 0.89 µM (ChTL); 34.4 versus 104.43 µM (TL); 2.95 versus 5.70 µM (PGPH), respectively).{17933}
Brand:CaymanSKU:-The ubiquitin-proteasome pathway plays an integral role in the selective degradation of intracellular proteins. While important for clearing damaged or mis-folded proteins, this proteolytic pathway also regulates the availability of key proteins involved in the control of inflammatory processes, cell cycle regulation, and gene expression.{13818,13345} (R)-MG132 is a potent, reversible, and cell permeable proteasome inhibitor. After treatment for one hour at 100 nM, it inhibits 50% and 31% of proteasome activity in lysates of J558L multiple myeloma cells and EMT6 breast cancer cells, respectively.{17933} The (R)-MG132 stereoisomer is a more effective inhibitor of chymotrypsin-like (ChTL), trypsin-like (TL), and peptidylglutamyl peptide hydrolyzing proteasome (PGPH) activities compared to (S)-MG132 (IC50s = 0.22 versus 0.89 µM (ChTL); 34.4 versus 104.43 µM (TL); 2.95 versus 5.70 µM (PGPH), respectively).{17933}
Brand:CaymanSKU:-The ubiquitin-proteasome pathway plays an integral role in the selective degradation of intracellular proteins. While important for clearing damaged or mis-folded proteins, this proteolytic pathway also regulates the availability of key proteins involved in the control of inflammatory processes, cell cycle regulation, and gene expression.{13818,13345} (R)-MG132 is a potent, reversible, and cell permeable proteasome inhibitor. After treatment for one hour at 100 nM, it inhibits 50% and 31% of proteasome activity in lysates of J558L multiple myeloma cells and EMT6 breast cancer cells, respectively.{17933} The (R)-MG132 stereoisomer is a more effective inhibitor of chymotrypsin-like (ChTL), trypsin-like (TL), and peptidylglutamyl peptide hydrolyzing proteasome (PGPH) activities compared to (S)-MG132 (IC50s = 0.22 versus 0.89 µM (ChTL); 34.4 versus 104.43 µM (TL); 2.95 versus 5.70 µM (PGPH), respectively).{17933}
Brand:CaymanSKU:-(R)-nitro-Blebbistatin is a more stable form of (+)-blebbistatin (Item No. 13165), which is the inactive form of (–)-blebbistatin (Item No. 13013). Prolonged exposure to blue light (450-490 nm) results in degradation of blebbistatin to an inactive product via cytotoxic intermediates, which may be problematic for its use in fluorescent live cell imaging applications.{17038,24037} The addition of a nitro group stabilizes the molecule to circumvent its degradation by prolonged blue light exposure.{16725} (R)-nitro-Blebbistatin has the same stereochemistry as the inactive (+)-blebbistatin enantiomer.
Brand:CaymanSKU:9001935 - 1 mgAvailable on backorder
(R)-nitro-Blebbistatin is a more stable form of (+)-blebbistatin (Item No. 13165), which is the inactive form of (–)-blebbistatin (Item No. 13013). Prolonged exposure to blue light (450-490 nm) results in degradation of blebbistatin to an inactive product via cytotoxic intermediates, which may be problematic for its use in fluorescent live cell imaging applications.{17038,24037} The addition of a nitro group stabilizes the molecule to circumvent its degradation by prolonged blue light exposure.{16725} (R)-nitro-Blebbistatin has the same stereochemistry as the inactive (+)-blebbistatin enantiomer.
Brand:CaymanSKU:9001935 - 10 mgAvailable on backorder
(R)-nitro-Blebbistatin is a more stable form of (+)-blebbistatin (Item No. 13165), which is the inactive form of (–)-blebbistatin (Item No. 13013). Prolonged exposure to blue light (450-490 nm) results in degradation of blebbistatin to an inactive product via cytotoxic intermediates, which may be problematic for its use in fluorescent live cell imaging applications.{17038,24037} The addition of a nitro group stabilizes the molecule to circumvent its degradation by prolonged blue light exposure.{16725} (R)-nitro-Blebbistatin has the same stereochemistry as the inactive (+)-blebbistatin enantiomer.
Brand:CaymanSKU:9001935 - 5 mgAvailable on backorder
(R)-Ofloxacin is a fluoroquinolone antibiotic and the (R) isomer of the antibiotics ofloxacin (Item No. 22891) and levofloxacin (Item No. 20382).{48800} It is active against certain Gram-positive and Gram-negative bacteria, including E. coli, P. aeruginosa strains 32104 and 32122, S. aureus strains 209P and Smith, and S. epidermis strain 56556 (MICs = 0.78, 12.5, 6.25, 25, 12.5, and 25 µg/ml, respectively) but not S. epidermis strain 56500, S. pyogenes, or S. faecalis (MICs = >100 µg/ml for all).{48801} (R)-Ofloxacin inhibits E. coli DNA gyrase with an IC50 value of 75 µg/ml, which is approximately 30- and 50-fold lower than inhibition by ofloxacin and levofloxacin, respectively.{48800}
Brand:CaymanSKU:29601 - 1 mgAvailable on backorder
(R)-Ofloxacin is a fluoroquinolone antibiotic and the (R) isomer of the antibiotics ofloxacin (Item No. 22891) and levofloxacin (Item No. 20382).{48800} It is active against certain Gram-positive and Gram-negative bacteria, including E. coli, P. aeruginosa strains 32104 and 32122, S. aureus strains 209P and Smith, and S. epidermis strain 56556 (MICs = 0.78, 12.5, 6.25, 25, 12.5, and 25 µg/ml, respectively) but not S. epidermis strain 56500, S. pyogenes, or S. faecalis (MICs = >100 µg/ml for all).{48801} (R)-Ofloxacin inhibits E. coli DNA gyrase with an IC50 value of 75 µg/ml, which is approximately 30- and 50-fold lower than inhibition by ofloxacin and levofloxacin, respectively.{48800}
Brand:CaymanSKU:29601 - 10 mgAvailable on backorder
(R)-Ofloxacin is a fluoroquinolone antibiotic and the (R) isomer of the antibiotics ofloxacin (Item No. 22891) and levofloxacin (Item No. 20382).{48800} It is active against certain Gram-positive and Gram-negative bacteria, including E. coli, P. aeruginosa strains 32104 and 32122, S. aureus strains 209P and Smith, and S. epidermis strain 56556 (MICs = 0.78, 12.5, 6.25, 25, 12.5, and 25 µg/ml, respectively) but not S. epidermis strain 56500, S. pyogenes, or S. faecalis (MICs = >100 µg/ml for all).{48801} (R)-Ofloxacin inhibits E. coli DNA gyrase with an IC50 value of 75 µg/ml, which is approximately 30- and 50-fold lower than inhibition by ofloxacin and levofloxacin, respectively.{48800}
Brand:CaymanSKU:29601 - 25 mgAvailable on backorder
(R)-Ofloxacin is a fluoroquinolone antibiotic and the (R) isomer of the antibiotics ofloxacin (Item No. 22891) and levofloxacin (Item No. 20382).{48800} It is active against certain Gram-positive and Gram-negative bacteria, including E. coli, P. aeruginosa strains 32104 and 32122, S. aureus strains 209P and Smith, and S. epidermis strain 56556 (MICs = 0.78, 12.5, 6.25, 25, 12.5, and 25 µg/ml, respectively) but not S. epidermis strain 56500, S. pyogenes, or S. faecalis (MICs = >100 µg/ml for all).{48801} (R)-Ofloxacin inhibits E. coli DNA gyrase with an IC50 value of 75 µg/ml, which is approximately 30- and 50-fold lower than inhibition by ofloxacin and levofloxacin, respectively.{48800}
Brand:CaymanSKU:29601 - 5 mgAvailable on backorder
(R)-Omeprazole is the inactive isomer of omeprazole (Item No. 14880), a gastric proton-pump inhibitor.{18249,28643} A stereoselective hydroxylation of (R)-omeprazole is mediated primarily by cytochrome P450 (CYP) 2C19, whereas CYP3A4 favors sulfoxidation of the active (S)-enantiomer (esomeprazole magnesium, Item No. 17326).{30673} (R)-Omeprazole has been shown to act as a reversible direct-acting and metabolism-dependent inhibitor of CYP2C19 in pooled human liver microsomes (IC50 = 8.1 µM).{30672}
Brand:CaymanSKU:-Available on backorder
(R)-Omeprazole is the inactive isomer of omeprazole (Item No. 14880), a gastric proton-pump inhibitor.{18249,28643} A stereoselective hydroxylation of (R)-omeprazole is mediated primarily by cytochrome P450 (CYP) 2C19, whereas CYP3A4 favors sulfoxidation of the active (S)-enantiomer (esomeprazole magnesium, Item No. 17326).{30673} (R)-Omeprazole has been shown to act as a reversible direct-acting and metabolism-dependent inhibitor of CYP2C19 in pooled human liver microsomes (IC50 = 8.1 µM).{30672}
Brand:CaymanSKU:-Available on backorder
(R)-Omeprazole is the inactive isomer of omeprazole (Item No. 14880), a gastric proton-pump inhibitor.{18249,28643} A stereoselective hydroxylation of (R)-omeprazole is mediated primarily by cytochrome P450 (CYP) 2C19, whereas CYP3A4 favors sulfoxidation of the active (S)-enantiomer (esomeprazole magnesium, Item No. 17326).{30673} (R)-Omeprazole has been shown to act as a reversible direct-acting and metabolism-dependent inhibitor of CYP2C19 in pooled human liver microsomes (IC50 = 8.1 µM).{30672}
Brand:CaymanSKU:-Available on backorder
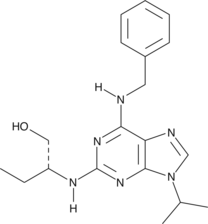
SET domain-containing protein 7/9 (SET7/9) is a histone methyltransferase that monomethylates lysine 4 of histone H3, which generates a specific tag for epigenetic transcriptional activation. It plays a role in the transcriptional activation of tumor suppressor p53 in response to DNA damage as well as the transcription factor TAF10.{16846,17223} (R)-PFI-2 is a potent, cell-permeable inhibitor of SET7/9 (IC50 = 2 nM) that demonstrates greater than 1,000-fold selectivity over a panel of 18 other methyltransferases.{26909} Its enantiomer, (S)-PFI-2 (Item No. 18119), is 500-fold less active (IC50 = 1 µM).{26909} See the Structural Genomics Consortium (SGC) website for more information.
Brand:CaymanSKU:-SET domain-containing protein 7/9 (SET7/9) is a histone methyltransferase that monomethylates lysine 4 of histone H3, which generates a specific tag for epigenetic transcriptional activation. It plays a role in the transcriptional activation of tumor suppressor p53 in response to DNA damage as well as the transcription factor TAF10.{16846,17223} (R)-PFI-2 is a potent, cell-permeable inhibitor of SET7/9 (IC50 = 2 nM) that demonstrates greater than 1,000-fold selectivity over a panel of 18 other methyltransferases.{26909} Its enantiomer, (S)-PFI-2 (Item No. 18119), is 500-fold less active (IC50 = 1 µM).{26909} See the Structural Genomics Consortium (SGC) website for more information.
Brand:CaymanSKU:-SET domain-containing protein 7/9 (SET7/9) is a histone methyltransferase that monomethylates lysine 4 of histone H3, which generates a specific tag for epigenetic transcriptional activation. It plays a role in the transcriptional activation of tumor suppressor p53 in response to DNA damage as well as the transcription factor TAF10.{16846,17223} (R)-PFI-2 is a potent, cell-permeable inhibitor of SET7/9 (IC50 = 2 nM) that demonstrates greater than 1,000-fold selectivity over a panel of 18 other methyltransferases.{26909} Its enantiomer, (S)-PFI-2 (Item No. 18119), is 500-fold less active (IC50 = 1 µM).{26909} See the Structural Genomics Consortium (SGC) website for more information.
Brand:CaymanSKU:-SET domain-containing protein 7/9 (SET7/9) is a histone methyltransferase that monomethylates lysine 4 of histone H3, which generates a specific tag for epigenetic transcriptional activation. It plays a role in the transcriptional activation of tumor suppressor p53 in response to DNA damage as well as the transcription factor TAF10.{16846,17223} (R)-PFI-2 is a potent, cell-permeable inhibitor of SET7/9 (IC50 = 2 nM) that demonstrates greater than 1,000-fold selectivity over a panel of 18 other methyltransferases.{26909} Its enantiomer, (S)-PFI-2 (Item No. 18119), is 500-fold less active (IC50 = 1 µM).{26909} See the Structural Genomics Consortium (SGC) website for more information.
Brand:CaymanSKU:-Pramipexole (Item No. 11981) is an agonist of dopamine receptors that has applications in Parkinson’s disease and other disorders.{21783,21784,21785,21786} Pramipexole is usually available as a mixture of enantiomers, with the majority of the dopamine receptor-dependent activity resulting from the (S) form.{29401} (R)-Pramipexole is an enantiomer of pramipexole that is ~100-fold less active than the (S) form as a dopamine receptor agonist.{29401,29399} For this reason, it can be used as a negative control for the (S) form in the study of dopamine receptors. Both isoforms are antioxidants that target mitochondria to prevent apoptosis.{29401,29402,29398,29403} This cytoprotective effect of (R)-pramipexole, without dopaminergic side effects, suggests utility in amyotrophic lateral sclerosis.{29401,29400}
Brand:CaymanSKU:-Available on backorder
Pramipexole (Item No. 11981) is an agonist of dopamine receptors that has applications in Parkinson’s disease and other disorders.{21783,21784,21785,21786} Pramipexole is usually available as a mixture of enantiomers, with the majority of the dopamine receptor-dependent activity resulting from the (S) form.{29401} (R)-Pramipexole is an enantiomer of pramipexole that is ~100-fold less active than the (S) form as a dopamine receptor agonist.{29401,29399} For this reason, it can be used as a negative control for the (S) form in the study of dopamine receptors. Both isoforms are antioxidants that target mitochondria to prevent apoptosis.{29401,29402,29398,29403} This cytoprotective effect of (R)-pramipexole, without dopaminergic side effects, suggests utility in amyotrophic lateral sclerosis.{29401,29400}
Brand:CaymanSKU:-Available on backorder
Pramipexole (Item No. 11981) is an agonist of dopamine receptors that has applications in Parkinson’s disease and other disorders.{21783,21784,21785,21786} Pramipexole is usually available as a mixture of enantiomers, with the majority of the dopamine receptor-dependent activity resulting from the (S) form.{29401} (R)-Pramipexole is an enantiomer of pramipexole that is ~100-fold less active than the (S) form as a dopamine receptor agonist.{29401,29399} For this reason, it can be used as a negative control for the (S) form in the study of dopamine receptors. Both isoforms are antioxidants that target mitochondria to prevent apoptosis.{29401,29402,29398,29403} This cytoprotective effect of (R)-pramipexole, without dopaminergic side effects, suggests utility in amyotrophic lateral sclerosis.{29401,29400}
Brand:CaymanSKU:-Available on backorder
Pramipexole (Item No. 11981) is an agonist of dopamine receptors that has applications in Parkinson’s disease and other disorders.{21783,21784,21785,21786} Pramipexole is usually available as a mixture of enantiomers, with the majority of the dopamine receptor-dependent activity resulting from the (S) form.{29401} (R)-Pramipexole is an enantiomer of pramipexole that is ~100-fold less active than the (S) form as a dopamine receptor agonist.{29401,29399} For this reason, it can be used as a negative control for the (S) form in the study of dopamine receptors. Both isoforms are antioxidants that target mitochondria to prevent apoptosis.{29401,29402,29398,29403} This cytoprotective effect of (R)-pramipexole, without dopaminergic side effects, suggests utility in amyotrophic lateral sclerosis.{29401,29400}
Brand:CaymanSKU:-Available on backorder
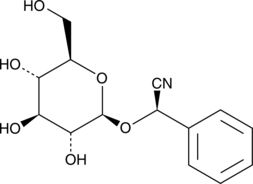
(R)-Prunasin is a cyanogenic glucoside that is the precursor to amygdalin, a bitter component found in species of the genera Prunus and Olinia.{25883} It can be degraded to hydrogen cyanide, glucose, and benzaldehyde by the action of prunasin hydrolase and mandelonitrile lyase and possesses inhibitory effects on plant growth.{25883} (R)-Prunasin reportedly inhibits rat DNA polymerase β, which performs base excision repair required for DNA maintenance and replication, with an IC50 value of 98 µM.{25882}
Brand:CaymanSKU:-(R)-Prunasin is a cyanogenic glucoside that is the precursor to amygdalin, a bitter component found in species of the genera Prunus and Olinia.{25883} It can be degraded to hydrogen cyanide, glucose, and benzaldehyde by the action of prunasin hydrolase and mandelonitrile lyase and possesses inhibitory effects on plant growth.{25883} (R)-Prunasin reportedly inhibits rat DNA polymerase β, which performs base excision repair required for DNA maintenance and replication, with an IC50 value of 98 µM.{25882}
Brand:CaymanSKU:-(R)-Prunasin is a cyanogenic glucoside that is the precursor to amygdalin, a bitter component found in species of the genera Prunus and Olinia.{25883} It can be degraded to hydrogen cyanide, glucose, and benzaldehyde by the action of prunasin hydrolase and mandelonitrile lyase and possesses inhibitory effects on plant growth.{25883} (R)-Prunasin reportedly inhibits rat DNA polymerase β, which performs base excision repair required for DNA maintenance and replication, with an IC50 value of 98 µM.{25882}
Brand:CaymanSKU:-Cyclin-dependent kinases (CDKs) are key regulators of cell cycle progression and are therefore promising targets for cancer therapy. (R)-Roscovitine is a potent inhibitor of Cdk2/cyclin E with an IC50 value of 0.1 µM.{14988} It also inhibits Cdk7/cyclin H, Cdk5/p35, and cell division cycle (cdc)/cyclin B with IC50 values of 0.49, 0.16, and 0.65 µM, respectively.{14987,14988,14991} (R)-Roscovitine inhibits the growth of rapidly proliferating cells with an average IC50 value of 15.2 µM against a panel of 19 human tumor cell lines.{14988} In murine models of polycystic kidney disease, (R)-roscovitine effectively inhibited disease progression at doses of 50-100 mg/kg.{14564}
Brand:CaymanSKU:10009569 - 1 mgAvailable on backorder
Cyclin-dependent kinases (CDKs) are key regulators of cell cycle progression and are therefore promising targets for cancer therapy. (R)-Roscovitine is a potent inhibitor of Cdk2/cyclin E with an IC50 value of 0.1 µM.{14988} It also inhibits Cdk7/cyclin H, Cdk5/p35, and cell division cycle (cdc)/cyclin B with IC50 values of 0.49, 0.16, and 0.65 µM, respectively.{14987,14988,14991} (R)-Roscovitine inhibits the growth of rapidly proliferating cells with an average IC50 value of 15.2 µM against a panel of 19 human tumor cell lines.{14988} In murine models of polycystic kidney disease, (R)-roscovitine effectively inhibited disease progression at doses of 50-100 mg/kg.{14564}
Brand:CaymanSKU:10009569 - 10 mgAvailable on backorder