Chemicals
Showing 3901–4050 of 41137 results
-
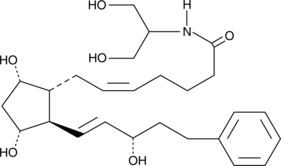
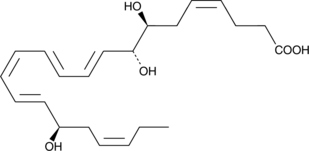
2-Arachidonyl glycerol (2-AG) exhibits cannabinoid agonist activity at the central cannabinoid (CB1) receptor, is an important endogenous monoglyceride species, and is thus considered to be the natural ligand for the CB1 receptor{5306,6819}. 2-AG can also be metabolized by COX-2 to form prostaglandin (PG) 2-glyceryl esters.{11045} These 2-glyceryl esters rapidly equilibrate to form 90:10 mixtures favoring the more stable 1-glyceryl ester. 17-phenyl trinor PGF2α serinol amide is a stable analog of PGF2α 2-glyceryl ester incorporating an activity-enhancing 17-phenyl substitution. The biological activity of 17-phenyl trinor PGF2α serinol amide has not yet been determined.
Brand:CaymanSKU:10004237 - 10 mgAvailable on backorder
2-Arachidonyl glycerol (2-AG) exhibits cannabinoid agonist activity at the central cannabinoid (CB1) receptor, is an important endogenous monoglyceride species, and is thus considered to be the natural ligand for the CB1 receptor{5306,6819}. 2-AG can also be metabolized by COX-2 to form prostaglandin (PG) 2-glyceryl esters.{11045} These 2-glyceryl esters rapidly equilibrate to form 90:10 mixtures favoring the more stable 1-glyceryl ester. 17-phenyl trinor PGF2α serinol amide is a stable analog of PGF2α 2-glyceryl ester incorporating an activity-enhancing 17-phenyl substitution. The biological activity of 17-phenyl trinor PGF2α serinol amide has not yet been determined.
Brand:CaymanSKU:10004237 - 5 mgAvailable on backorder
2-Arachidonyl glycerol (2-AG) exhibits cannabinoid agonist activity at the central cannabinoid (CB1) receptor, is an important endogenous monoglyceride species, and is thus considered to be the natural ligand for the CB1 receptor{5306,6819}. 2-AG can also be metabolized by COX-2 to form prostaglandin (PG) 2-glyceryl esters.{11045} These 2-glyceryl esters rapidly equilibrate to form 90:10 mixtures favoring the more stable 1-glyceryl ester. 17-phenyl trinor PGF2α serinol amide is a stable analog of PGF2α 2-glyceryl ester incorporating an activity-enhancing 17-phenyl substitution. The biological activity of 17-phenyl trinor PGF2α serinol amide has not yet been determined.
Brand:CaymanSKU:10004237 - 50 mgAvailable on backorder
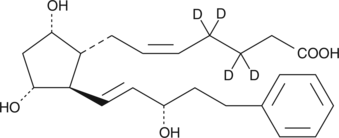
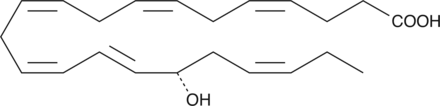
17-phenyl trinor Prostaglandin F2α-d4 (17-phenyl trinor PGF2α-d4) contains four deuterium atoms at the 3, 3′, 4, and 4′ positions. It is intended for use as an internal standard for the quantification of 17-phenyl trinor PGF2α by GC- or LC-mass spectrometry. 17-phenyl trinor PGF2α is a metabolically stable analog of PGF2α and is a potent agonist for the FP receptor. It binds to the FP receptor on ovine luteal cells with a relative potency of 756% compared to that of PGF2α.{2058} At the rat recombinant FP receptor expressed in CHO cells 17-phenyl trinor PGF2α inhibits PGF2α binding with a Ki of 1.1 nM.{1374} The isopropyl ester of 17-phenyl trinor PGF2α-d4 is slightly better than PGF2α isopropyl ester in reducing the intraocular pressure in the cat eye without any irritation.{1107}
Brand:CaymanSKU:316810 - 1 mgAvailable on backorder
17-phenyl trinor Prostaglandin F2α-d4 (17-phenyl trinor PGF2α-d4) contains four deuterium atoms at the 3, 3′, 4, and 4′ positions. It is intended for use as an internal standard for the quantification of 17-phenyl trinor PGF2α by GC- or LC-mass spectrometry. 17-phenyl trinor PGF2α is a metabolically stable analog of PGF2α and is a potent agonist for the FP receptor. It binds to the FP receptor on ovine luteal cells with a relative potency of 756% compared to that of PGF2α.{2058} At the rat recombinant FP receptor expressed in CHO cells 17-phenyl trinor PGF2α inhibits PGF2α binding with a Ki of 1.1 nM.{1374} The isopropyl ester of 17-phenyl trinor PGF2α-d4 is slightly better than PGF2α isopropyl ester in reducing the intraocular pressure in the cat eye without any irritation.{1107}
Brand:CaymanSKU:316810 - 100 µgAvailable on backorder
17-phenyl trinor Prostaglandin F2α-d4 (17-phenyl trinor PGF2α-d4) contains four deuterium atoms at the 3, 3′, 4, and 4′ positions. It is intended for use as an internal standard for the quantification of 17-phenyl trinor PGF2α by GC- or LC-mass spectrometry. 17-phenyl trinor PGF2α is a metabolically stable analog of PGF2α and is a potent agonist for the FP receptor. It binds to the FP receptor on ovine luteal cells with a relative potency of 756% compared to that of PGF2α.{2058} At the rat recombinant FP receptor expressed in CHO cells 17-phenyl trinor PGF2α inhibits PGF2α binding with a Ki of 1.1 nM.{1374} The isopropyl ester of 17-phenyl trinor PGF2α-d4 is slightly better than PGF2α isopropyl ester in reducing the intraocular pressure in the cat eye without any irritation.{1107}
Brand:CaymanSKU:316810 - 25 µgAvailable on backorder
17-phenyl trinor Prostaglandin F2α-d4 (17-phenyl trinor PGF2α-d4) contains four deuterium atoms at the 3, 3′, 4, and 4′ positions. It is intended for use as an internal standard for the quantification of 17-phenyl trinor PGF2α by GC- or LC-mass spectrometry. 17-phenyl trinor PGF2α is a metabolically stable analog of PGF2α and is a potent agonist for the FP receptor. It binds to the FP receptor on ovine luteal cells with a relative potency of 756% compared to that of PGF2α.{2058} At the rat recombinant FP receptor expressed in CHO cells 17-phenyl trinor PGF2α inhibits PGF2α binding with a Ki of 1.1 nM.{1374} The isopropyl ester of 17-phenyl trinor PGF2α-d4 is slightly better than PGF2α isopropyl ester in reducing the intraocular pressure in the cat eye without any irritation.{1107}
Brand:CaymanSKU:316810 - 50 µgAvailable on backorder
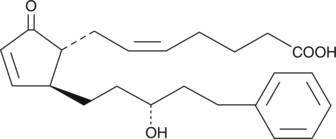
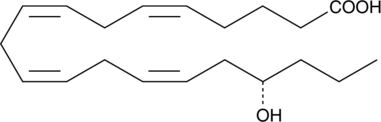
17-phenyl trinor-13,14-dihydro Prostaglandin A2 is a synthetic PG analog whose biological activity has not been widely reported. The PGF2. 17-phenyl trinor-13,14-dihydro Prostaglandin A2 is a synthetic PG analog whose biological activity has not been widely reported. The PGF2.
Brand:CaymanSKU:10290 - 1 mgAvailable on backorder
17-phenyl trinor-13,14-dihydro Prostaglandin A2 is a synthetic PG analog whose biological activity has not been widely reported. The PGF2. 17-phenyl trinor-13,14-dihydro Prostaglandin A2 is a synthetic PG analog whose biological activity has not been widely reported. The PGF2.
Brand:CaymanSKU:10290 - 10 mgAvailable on backorder
17-phenyl trinor-13,14-dihydro Prostaglandin A2 is a synthetic PG analog whose biological activity has not been widely reported. The PGF2. 17-phenyl trinor-13,14-dihydro Prostaglandin A2 is a synthetic PG analog whose biological activity has not been widely reported. The PGF2.
Brand:CaymanSKU:10290 - 5 mgAvailable on backorder
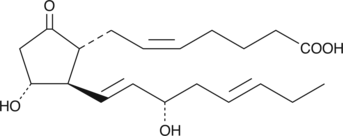
17-trans PGE3 is an isomer of PGE3 which could theoretically result from the COX metabolism of dietary trans-fatty acids.{5470} There are no published reports on the biological activity of this compound.
Brand:CaymanSKU:-17-trans PGE3 is an isomer of PGE3 which could theoretically result from the COX metabolism of dietary trans-fatty acids.{5470} There are no published reports on the biological activity of this compound.
Brand:CaymanSKU:-17-trans PGE3 is an isomer of PGE3 which could theoretically result from the COX metabolism of dietary trans-fatty acids.{5470} There are no published reports on the biological activity of this compound.
Brand:CaymanSKU:-17-trans PGF3α is a double bond isomer of PGF3α and a potential metabolite of trans dietary fatty acids. There are no published reports on the biological activity of this compound.
Brand:CaymanSKU:-Out of stock
17-trans PGF3α is a double bond isomer of PGF3α and a potential metabolite of trans dietary fatty acids. There are no published reports on the biological activity of this compound.
Brand:CaymanSKU:-Out of stock
17-trans PGF3α is a double bond isomer of PGF3α and a potential metabolite of trans dietary fatty acids. There are no published reports on the biological activity of this compound.
Brand:CaymanSKU:-Out of stock
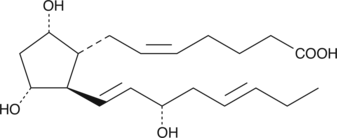
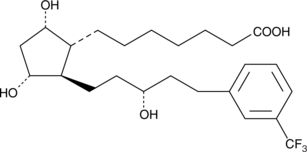
A number of 17-phenyl trinor prostaglandin F2α (17-phenyl trinor PGF2α) derivatives have been approved for the treatment of glaucoma.{8941,9341,8839} Of these, the unsubstituted or meta-substituted aromatic derivatives are the most potent FP receptor agonists.{9668} 17-trifluoromethylphenyl trinor PGF2α bears an aromatic ring which is reminiscent of the trifluoromethyl-phenoxy ring of travoprost ((+)-fluprostenol isopropyl ester). As an ocular hypotensive agent, it would be expected that 17-trifluoromethylphenyl trinor PGF2α would act very much like the free acid of travoprost. 17-phenyl trinor PGF2α is a potent luteolytic and abortifacient, with a potency equal to or greater than fluprostenol and cloprostenol.{9668}
Brand:CaymanSKU:-Out of stock
A number of 17-phenyl trinor prostaglandin F2α (17-phenyl trinor PGF2α) derivatives have been approved for the treatment of glaucoma.{8941,9341,8839} Of these, the unsubstituted or meta-substituted aromatic derivatives are the most potent FP receptor agonists.{9668} 17-trifluoromethylphenyl trinor PGF2α bears an aromatic ring which is reminiscent of the trifluoromethyl-phenoxy ring of travoprost ((+)-fluprostenol isopropyl ester). As an ocular hypotensive agent, it would be expected that 17-trifluoromethylphenyl trinor PGF2α would act very much like the free acid of travoprost. 17-phenyl trinor PGF2α is a potent luteolytic and abortifacient, with a potency equal to or greater than fluprostenol and cloprostenol.{9668}
Brand:CaymanSKU:-Out of stock
A number of 17-phenyl trinor prostaglandin F2α (17-phenyl trinor PGF2α) derivatives have been approved for the treatment of glaucoma.{8941,9341,8839} Of these, the unsubstituted or meta-substituted aromatic derivatives are the most potent FP receptor agonists.{9668} 17-trifluoromethylphenyl trinor PGF2α bears an aromatic ring which is reminiscent of the trifluoromethyl-phenoxy ring of travoprost ((+)-fluprostenol isopropyl ester). As an ocular hypotensive agent, it would be expected that 17-trifluoromethylphenyl trinor PGF2α would act very much like the free acid of travoprost. 17-phenyl trinor PGF2α is a potent luteolytic and abortifacient, with a potency equal to or greater than fluprostenol and cloprostenol.{9668}
Brand:CaymanSKU:-Out of stock
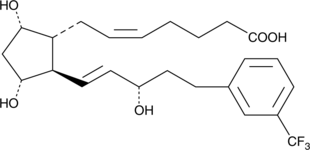
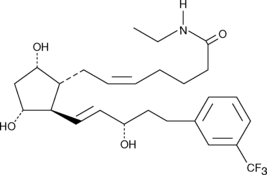
Prostaglandin F2α (PGF2α), acting through the FP receptor, causes smooth muscle contraction and exhibits potent luteolytic activity.{187,421,1651} 17-trifluoromethylphenyl trinor Prostaglandin F2α (17-trifluoromethylphenyl trinor PGF2α) is an analog of PGF2α that shares the meta-trifluoromethyl group of travoprost with the 17-phenyl trinor modification of latanoprost. It is anticipated to be a potent and selective agonist of the FP receptor, with potential applications in glaucoma and luteolysis. 17-trifluoromethylphenyl trinor PGF2α ethyl amide is a lipophilic analog of 17-trifluoromethylphenyl trinor PGF2α. Ethyl amides of PGs can serve as prodrugs, as they are hydrolyzed in certain tissues to generate the bioactive free acid.
Brand:CaymanSKU:10010061 - 1 mgAvailable on backorder
Prostaglandin F2α (PGF2α), acting through the FP receptor, causes smooth muscle contraction and exhibits potent luteolytic activity.{187,421,1651} 17-trifluoromethylphenyl trinor Prostaglandin F2α (17-trifluoromethylphenyl trinor PGF2α) is an analog of PGF2α that shares the meta-trifluoromethyl group of travoprost with the 17-phenyl trinor modification of latanoprost. It is anticipated to be a potent and selective agonist of the FP receptor, with potential applications in glaucoma and luteolysis. 17-trifluoromethylphenyl trinor PGF2α ethyl amide is a lipophilic analog of 17-trifluoromethylphenyl trinor PGF2α. Ethyl amides of PGs can serve as prodrugs, as they are hydrolyzed in certain tissues to generate the bioactive free acid.
Brand:CaymanSKU:10010061 - 10 mgAvailable on backorder
Prostaglandin F2α (PGF2α), acting through the FP receptor, causes smooth muscle contraction and exhibits potent luteolytic activity.{187,421,1651} 17-trifluoromethylphenyl trinor Prostaglandin F2α (17-trifluoromethylphenyl trinor PGF2α) is an analog of PGF2α that shares the meta-trifluoromethyl group of travoprost with the 17-phenyl trinor modification of latanoprost. It is anticipated to be a potent and selective agonist of the FP receptor, with potential applications in glaucoma and luteolysis. 17-trifluoromethylphenyl trinor PGF2α ethyl amide is a lipophilic analog of 17-trifluoromethylphenyl trinor PGF2α. Ethyl amides of PGs can serve as prodrugs, as they are hydrolyzed in certain tissues to generate the bioactive free acid.
Brand:CaymanSKU:10010061 - 5 mgAvailable on backorder
Prostaglandin F2α (PGF2α), acting through the FP receptor, causes smooth muscle contraction and exhibits potent luteolytic activity.{187,421,1651} 17-trifluoromethylphenyl trinor Prostaglandin F2α (17-trifluoromethylphenyl trinor PGF2α) is an analog of PGF2α that shares the meta-trifluoromethyl group of travoprost with the 17-phenyl trinor modification of latanoprost. It is anticipated to be a potent and selective agonist of the FP receptor, with potential applications in glaucoma and luteolysis. 17-trifluoromethylphenyl trinor PGF2α ethyl amide is a lipophilic analog of 17-trifluoromethylphenyl trinor PGF2α. Ethyl amides of PGs can serve as prodrugs, as they are hydrolyzed in certain tissues to generate the bioactive free acid.
Brand:CaymanSKU:10010061 - 50 mgAvailable on backorder
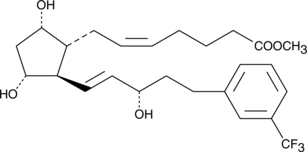
Prostaglandin F2α (PGF2α), acting through the FP receptor, causes smooth muscle contraction and exhibits potent luteolytic activity.{187,421,1651} 17-trifluoromethylphenyl trinor PGF2α is an analog of PGF2α that shares the meta-trifluoromethyl group of travoprost with the 17-phenyl trinor modification of latanoprost. It is anticipated to be a potent and selective agonist of the FP receptor, with potential applications in glaucoma and luteolysis. 17-trifluoromethylphenyl trinor PGF2α methyl ester is a lipophilic analog of 17-trifluoromethylphenyl trinor PGF2α. Methyl esters of PGs serve as prodrugs, as they are efficiently hydrolyzed in certain tissues to generate the bioactive free acid.
Brand:CaymanSKU:10010111 - 1 mgAvailable on backorder
Prostaglandin F2α (PGF2α), acting through the FP receptor, causes smooth muscle contraction and exhibits potent luteolytic activity.{187,421,1651} 17-trifluoromethylphenyl trinor PGF2α is an analog of PGF2α that shares the meta-trifluoromethyl group of travoprost with the 17-phenyl trinor modification of latanoprost. It is anticipated to be a potent and selective agonist of the FP receptor, with potential applications in glaucoma and luteolysis. 17-trifluoromethylphenyl trinor PGF2α methyl ester is a lipophilic analog of 17-trifluoromethylphenyl trinor PGF2α. Methyl esters of PGs serve as prodrugs, as they are efficiently hydrolyzed in certain tissues to generate the bioactive free acid.
Brand:CaymanSKU:10010111 - 10 mgAvailable on backorder
Prostaglandin F2α (PGF2α), acting through the FP receptor, causes smooth muscle contraction and exhibits potent luteolytic activity.{187,421,1651} 17-trifluoromethylphenyl trinor PGF2α is an analog of PGF2α that shares the meta-trifluoromethyl group of travoprost with the 17-phenyl trinor modification of latanoprost. It is anticipated to be a potent and selective agonist of the FP receptor, with potential applications in glaucoma and luteolysis. 17-trifluoromethylphenyl trinor PGF2α methyl ester is a lipophilic analog of 17-trifluoromethylphenyl trinor PGF2α. Methyl esters of PGs serve as prodrugs, as they are efficiently hydrolyzed in certain tissues to generate the bioactive free acid.
Brand:CaymanSKU:10010111 - 5 mgAvailable on backorder
A number of 17-aryl trinor and 16-aryloxy tetranor prostaglandin F2α derivatives have been approved for the treatment of glaucoma.{8941,9341,8839} These “ring” prostaglandin (PG) analogs have improved efficacy over the PGs with an n-alkyl lower side chain. Of these, the ones wherein the 13,14-double bond has been hydrogenated retain relatively good potency, but show a significantly reduced incidence of local irritant side effects.{9736} 17-trifluoromethylphenyl-13,14-dihydro trinor PGF1α (17-TFM-PGF1α) is a typical “ring” analog reminiscent of the trifluoromethyl-phenoxy ring of Travoprost. The a chain of 17-TFM-PGF1α is saturated, making this compound a formal member of the one-series PGs. Recent work has shown that in the “ring” series of analogs, this modification has little impact on FP receptor binding.{9668} As an ocular hypotensive agent, it is expected that 17-TFM-PGF1α would act very much like the free acid of latanoprost.
Brand:CaymanSKU:-A number of 17-aryl trinor and 16-aryloxy tetranor prostaglandin F2α derivatives have been approved for the treatment of glaucoma.{8941,9341,8839} These “ring” prostaglandin (PG) analogs have improved efficacy over the PGs with an n-alkyl lower side chain. Of these, the ones wherein the 13,14-double bond has been hydrogenated retain relatively good potency, but show a significantly reduced incidence of local irritant side effects.{9736} 17-trifluoromethylphenyl-13,14-dihydro trinor PGF1α (17-TFM-PGF1α) is a typical “ring” analog reminiscent of the trifluoromethyl-phenoxy ring of Travoprost. The a chain of 17-TFM-PGF1α is saturated, making this compound a formal member of the one-series PGs. Recent work has shown that in the “ring” series of analogs, this modification has little impact on FP receptor binding.{9668} As an ocular hypotensive agent, it is expected that 17-TFM-PGF1α would act very much like the free acid of latanoprost.
Brand:CaymanSKU:-A number of 17-aryl trinor and 16-aryloxy tetranor prostaglandin F2α derivatives have been approved for the treatment of glaucoma.{8941,9341,8839} These “ring” prostaglandin (PG) analogs have improved efficacy over the PGs with an n-alkyl lower side chain. Of these, the ones wherein the 13,14-double bond has been hydrogenated retain relatively good potency, but show a significantly reduced incidence of local irritant side effects.{9736} 17-trifluoromethylphenyl-13,14-dihydro trinor PGF1α (17-TFM-PGF1α) is a typical “ring” analog reminiscent of the trifluoromethyl-phenoxy ring of Travoprost. The a chain of 17-TFM-PGF1α is saturated, making this compound a formal member of the one-series PGs. Recent work has shown that in the “ring” series of analogs, this modification has little impact on FP receptor binding.{9668} As an ocular hypotensive agent, it is expected that 17-TFM-PGF1α would act very much like the free acid of latanoprost.
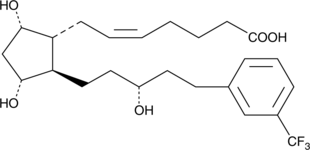
Brand:CaymanSKU:-A number of 17-phenyl trinor prostaglandin F2α (17-phenyl trinor PGF2α) derivatives have been approved for the treatment of glaucoma.{8941,9341,8839} Of these, the ones wherein the 13,14-double bond has been hydrogenated retain relatively good potency, but show a significantly reduced incidence of local irritant side effects.{9736} 17-trifluoromethylphenyl-13,14-dihydro trinor PGF2α bears an aromatic ring which is reminiscent of the trifluoromethyl-phenoxy ring of travoprost ((+)-fluprostenol isopropyl ester). As an ocular hypotensive agent, it would be expected that 17-trifluoromethylphenyl-13,14-dihydro trinor PGF2α would act very much like the free acid of latanoprost.
Brand:CaymanSKU:-Out of stock
A number of 17-phenyl trinor prostaglandin F2α (17-phenyl trinor PGF2α) derivatives have been approved for the treatment of glaucoma.{8941,9341,8839} Of these, the ones wherein the 13,14-double bond has been hydrogenated retain relatively good potency, but show a significantly reduced incidence of local irritant side effects.{9736} 17-trifluoromethylphenyl-13,14-dihydro trinor PGF2α bears an aromatic ring which is reminiscent of the trifluoromethyl-phenoxy ring of travoprost ((+)-fluprostenol isopropyl ester). As an ocular hypotensive agent, it would be expected that 17-trifluoromethylphenyl-13,14-dihydro trinor PGF2α would act very much like the free acid of latanoprost.
Brand:CaymanSKU:-Out of stock
A number of 17-phenyl trinor prostaglandin F2α (17-phenyl trinor PGF2α) derivatives have been approved for the treatment of glaucoma.{8941,9341,8839} Of these, the ones wherein the 13,14-double bond has been hydrogenated retain relatively good potency, but show a significantly reduced incidence of local irritant side effects.{9736} 17-trifluoromethylphenyl-13,14-dihydro trinor PGF2α bears an aromatic ring which is reminiscent of the trifluoromethyl-phenoxy ring of travoprost ((+)-fluprostenol isopropyl ester). As an ocular hypotensive agent, it would be expected that 17-trifluoromethylphenyl-13,14-dihydro trinor PGF2α would act very much like the free acid of latanoprost.
Brand:CaymanSKU:-Out of stock
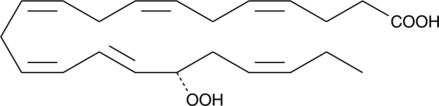
Resolvins are a group of polyhydroxylated metabolites of docosahexaenoic acid (DHA) found in the inflammatory exudates of aspirin-treated experimental animals.{11433} 17(R)-HDHA is the primary oxygenation product of DHA when exposed to aspirin-inhibited cyclooxygenase-2. 17(R)-HDHA serves as a precursor to resolvins and has intrinsic biological activity, such as the inhibition of TNFα-induced IL-1β expression in human glioma cells.{11433}
Brand:CaymanSKU:10005099 - 100 µgAvailable on backorder
Resolvins are a group of polyhydroxylated metabolites of docosahexaenoic acid (DHA) found in the inflammatory exudates of aspirin-treated experimental animals.{11433} 17(R)-HDHA is the primary oxygenation product of DHA when exposed to aspirin-inhibited cyclooxygenase-2. 17(R)-HDHA serves as a precursor to resolvins and has intrinsic biological activity, such as the inhibition of TNFα-induced IL-1β expression in human glioma cells.{11433}
Brand:CaymanSKU:10005099 - 25 µgAvailable on backorder
Resolvins are a group of polyhydroxylated metabolites of docosahexaenoic acid (DHA) found in the inflammatory exudates of aspirin-treated experimental animals.{11433} 17(R)-HDHA is the primary oxygenation product of DHA when exposed to aspirin-inhibited cyclooxygenase-2. 17(R)-HDHA serves as a precursor to resolvins and has intrinsic biological activity, such as the inhibition of TNFα-induced IL-1β expression in human glioma cells.{11433}
Brand:CaymanSKU:10005099 - 250 µgAvailable on backorder
Resolvins are a group of polyhydroxylated metabolites of docosahexaenoic acid (DHA) found in the inflammatory exudates of aspirin-treated experimental animals.{11433} 17(R)-HDHA is the primary oxygenation product of DHA when exposed to aspirin-inhibited cyclooxygenase-2. 17(R)-HDHA serves as a precursor to resolvins and has intrinsic biological activity, such as the inhibition of TNFα-induced IL-1β expression in human glioma cells.{11433}
Brand:CaymanSKU:10005099 - 50 µgAvailable on backorder
Electrolyte and fluid transport in the kidney are regulated in part by arachidonic acid and its metabolites. Electrolyte and fluid transport in the kidney are regulated in part by arachidonic acid and its metabolites. 17-HETE is a cytochrome P450 (CYP450) metabolite of arachidonic acid that has stereospecific effects on sodium transport in the kidney. 17(R)-HETE is an inactive isomer of 17-HETE, whereas the (S) enantiomer can inhibit proximal tubule ATPase activity at a concentration of 2 µM. {3825}
Brand:CaymanSKU:10010637 - 100 µgAvailable on backorder
Electrolyte and fluid transport in the kidney are regulated in part by arachidonic acid and its metabolites. Electrolyte and fluid transport in the kidney are regulated in part by arachidonic acid and its metabolites. 17-HETE is a cytochrome P450 (CYP450) metabolite of arachidonic acid that has stereospecific effects on sodium transport in the kidney. 17(R)-HETE is an inactive isomer of 17-HETE, whereas the (S) enantiomer can inhibit proximal tubule ATPase activity at a concentration of 2 µM. {3825}
Brand:CaymanSKU:10010637 - 25 µgAvailable on backorder
Electrolyte and fluid transport in the kidney are regulated in part by arachidonic acid and its metabolites. Electrolyte and fluid transport in the kidney are regulated in part by arachidonic acid and its metabolites. 17-HETE is a cytochrome P450 (CYP450) metabolite of arachidonic acid that has stereospecific effects on sodium transport in the kidney. 17(R)-HETE is an inactive isomer of 17-HETE, whereas the (S) enantiomer can inhibit proximal tubule ATPase activity at a concentration of 2 µM. {3825}
Brand:CaymanSKU:10010637 - 50 µgAvailable on backorder
Resolvins are a family of potent lipid mediators derived from both eicosapentaenoic acid (EPA; Item No. 90110) and docosahexaenoic acid (DHA; Item No. 90310).{13989} In addition to being anti-inflammatory, resolvins promote the resolution of the inflammatory response back to a non-inflamed state.{14973} Resolvin D1 (RvD1; Item No. 10012554) is produced physiologically from the sequential oxygenation of DHA by 15- and 5-lipoxygenase.{13989} 17(R)-RvD1 is an aspirin-triggered epimer of RvD1 that reduces human polymorphonuclear leukocyte (PMN) transendothelial migration, the earliest event in acute inflammation, with equipotency to RvD1 (EC50 = ~30 nM).{15718} 17(R)-RvD1 exhibits a dose-dependent reduction in leukocyte infiltration in a mouse model of peritonitis with maximal inhibition of ~35% at a 100 ng dose.{15718} In contrast to RvD1, the aspirin-triggered form resists rapid inactivation by eicosanoid oxidoreductases. Analytical and biological comparisons of synthetic 17(R)-RvD1 with endogenously derived 17(R)-RvD1 have confirmed its identity as matching the natural product.{14569}
Brand:CaymanSKU:13060 - 10 µgAvailable on backorder
Resolvins are a family of potent lipid mediators derived from both eicosapentaenoic acid (EPA; Item No. 90110) and docosahexaenoic acid (DHA; Item No. 90310).{13989} In addition to being anti-inflammatory, resolvins promote the resolution of the inflammatory response back to a non-inflamed state.{14973} Resolvin D1 (RvD1; Item No. 10012554) is produced physiologically from the sequential oxygenation of DHA by 15- and 5-lipoxygenase.{13989} 17(R)-RvD1 is an aspirin-triggered epimer of RvD1 that reduces human polymorphonuclear leukocyte (PMN) transendothelial migration, the earliest event in acute inflammation, with equipotency to RvD1 (EC50 = ~30 nM).{15718} 17(R)-RvD1 exhibits a dose-dependent reduction in leukocyte infiltration in a mouse model of peritonitis with maximal inhibition of ~35% at a 100 ng dose.{15718} In contrast to RvD1, the aspirin-triggered form resists rapid inactivation by eicosanoid oxidoreductases. Analytical and biological comparisons of synthetic 17(R)-RvD1 with endogenously derived 17(R)-RvD1 have confirmed its identity as matching the natural product.{14569}
Brand:CaymanSKU:13060 - 100 µgAvailable on backorder
Resolvins are a family of potent lipid mediators derived from both eicosapentaenoic acid (EPA; Item No. 90110) and docosahexaenoic acid (DHA; Item No. 90310).{13989} In addition to being anti-inflammatory, resolvins promote the resolution of the inflammatory response back to a non-inflamed state.{14973} Resolvin D1 (RvD1; Item No. 10012554) is produced physiologically from the sequential oxygenation of DHA by 15- and 5-lipoxygenase.{13989} 17(R)-RvD1 is an aspirin-triggered epimer of RvD1 that reduces human polymorphonuclear leukocyte (PMN) transendothelial migration, the earliest event in acute inflammation, with equipotency to RvD1 (EC50 = ~30 nM).{15718} 17(R)-RvD1 exhibits a dose-dependent reduction in leukocyte infiltration in a mouse model of peritonitis with maximal inhibition of ~35% at a 100 ng dose.{15718} In contrast to RvD1, the aspirin-triggered form resists rapid inactivation by eicosanoid oxidoreductases. Analytical and biological comparisons of synthetic 17(R)-RvD1 with endogenously derived 17(R)-RvD1 have confirmed its identity as matching the natural product.{14569}
Brand:CaymanSKU:13060 - 25 µgAvailable on backorder
Resolvins are a family of potent lipid mediators derived from both eicosapentaenoic acid (EPA; Item No. 90110) and docosahexaenoic acid (DHA; Item No. 90310).{13989} In addition to being anti-inflammatory, resolvins promote the resolution of the inflammatory response back to a non-inflamed state.{14973} Resolvin D1 (RvD1; Item No. 10012554) is produced physiologically from the sequential oxygenation of DHA by 15- and 5-lipoxygenase.{13989} 17(R)-RvD1 is an aspirin-triggered epimer of RvD1 that reduces human polymorphonuclear leukocyte (PMN) transendothelial migration, the earliest event in acute inflammation, with equipotency to RvD1 (EC50 = ~30 nM).{15718} 17(R)-RvD1 exhibits a dose-dependent reduction in leukocyte infiltration in a mouse model of peritonitis with maximal inhibition of ~35% at a 100 ng dose.{15718} In contrast to RvD1, the aspirin-triggered form resists rapid inactivation by eicosanoid oxidoreductases. Analytical and biological comparisons of synthetic 17(R)-RvD1 with endogenously derived 17(R)-RvD1 have confirmed its identity as matching the natural product.{14569}
Brand:CaymanSKU:13060 - 50 µgAvailable on backorder
17(R)-Resolvin D1 (17(R)-RvD1) is an aspirin-triggered epimer of RvD1 (Item No. 10012554) that reduces human polymorphonuclear leukocyte transendothelial migration, the earliest event in acute inflammation, with equipotency to RvD1 (EC50 = ~30 nM).{15718} 17(R)-RvD1 exhibits a dose-dependent reduction in leukocyte infiltration in a mouse model of peritonitis with maximal inhibition of ~35% at a 100 ng dose.{15718} In contrast to RvD1, the aspirin-triggered form resists rapid inactivation by eicosanoid oxidoreductases. 17(R)-RvD1 methyl ester is a methyl ester version of the free acid which may act as a lipophilic prodrug form that will alter its distribution and pharmacokinetic properties. The methyl ester moiety is susceptible to cleavage by intracellular esterases, leaving the free acid.
Brand:CaymanSKU:9001072 - 10 µgAvailable on backorder
17(R)-Resolvin D1 (17(R)-RvD1) is an aspirin-triggered epimer of RvD1 (Item No. 10012554) that reduces human polymorphonuclear leukocyte transendothelial migration, the earliest event in acute inflammation, with equipotency to RvD1 (EC50 = ~30 nM).{15718} 17(R)-RvD1 exhibits a dose-dependent reduction in leukocyte infiltration in a mouse model of peritonitis with maximal inhibition of ~35% at a 100 ng dose.{15718} In contrast to RvD1, the aspirin-triggered form resists rapid inactivation by eicosanoid oxidoreductases. 17(R)-RvD1 methyl ester is a methyl ester version of the free acid which may act as a lipophilic prodrug form that will alter its distribution and pharmacokinetic properties. The methyl ester moiety is susceptible to cleavage by intracellular esterases, leaving the free acid.
Brand:CaymanSKU:9001072 - 100 µgAvailable on backorder
17(R)-Resolvin D1 (17(R)-RvD1) is an aspirin-triggered epimer of RvD1 (Item No. 10012554) that reduces human polymorphonuclear leukocyte transendothelial migration, the earliest event in acute inflammation, with equipotency to RvD1 (EC50 = ~30 nM).{15718} 17(R)-RvD1 exhibits a dose-dependent reduction in leukocyte infiltration in a mouse model of peritonitis with maximal inhibition of ~35% at a 100 ng dose.{15718} In contrast to RvD1, the aspirin-triggered form resists rapid inactivation by eicosanoid oxidoreductases. 17(R)-RvD1 methyl ester is a methyl ester version of the free acid which may act as a lipophilic prodrug form that will alter its distribution and pharmacokinetic properties. The methyl ester moiety is susceptible to cleavage by intracellular esterases, leaving the free acid.
Brand:CaymanSKU:9001072 - 25 µgAvailable on backorder
17(R)-Resolvin D1 (17(R)-RvD1) is an aspirin-triggered epimer of RvD1 (Item No. 10012554) that reduces human polymorphonuclear leukocyte transendothelial migration, the earliest event in acute inflammation, with equipotency to RvD1 (EC50 = ~30 nM).{15718} 17(R)-RvD1 exhibits a dose-dependent reduction in leukocyte infiltration in a mouse model of peritonitis with maximal inhibition of ~35% at a 100 ng dose.{15718} In contrast to RvD1, the aspirin-triggered form resists rapid inactivation by eicosanoid oxidoreductases. 17(R)-RvD1 methyl ester is a methyl ester version of the free acid which may act as a lipophilic prodrug form that will alter its distribution and pharmacokinetic properties. The methyl ester moiety is susceptible to cleavage by intracellular esterases, leaving the free acid.
Brand:CaymanSKU:9001072 - 50 µgAvailable on backorder
17(R)-Resolvin D3 (17(R)-RvD3) is an aspirin-triggered epimer of resolvin D3 (Item No. 13834).{25262} It is produced from docosahexaenoic acid (DHA; Item No. 90310) by COX-2 in the presence of aspirin via a 17(R)-hydroperoxy DHA (17(R)-HDHA; Item No. 10005099) intermediate and has been found in mouse inflammatory exudates.{25262,45355} 17(R)-RvD3 reduces transmigration of isolated human polymorphonuclear cells (PMNs) and induces efferocytosis of apoptotic PMNs by macrophages.{45355} 17(R)-RvD3 (10 ng/animal) reduces transmigration of neutrophils into the peritoneal cavity, as well as decreases the levels of IL-6 and increases the levels of IL-10 in inflammatory exudate in a mouse model of zymosan-induced peritonitis. It activates GPR32 in a β-arrestin reporter assay and increases phagocytosis to a greater degree in CHO cells overexpressing GPR32 compared to mock-transfected cells. 17(R)-RvD3 increases phagocytosis of etoposide-generated tumor cell debris by monocyte-derived macrophages in H460 human lung carcinoma cells in a concentration-dependent manner.{45356} It also inhibits tumor growth in a mouse model of Lewis lung carcinoma when administered at a dose of 0.6 µg/kg per day.
Brand:CaymanSKU:9002880 - 10 µgAvailable on backorder
17(R)-Resolvin D3 (17(R)-RvD3) is an aspirin-triggered epimer of resolvin D3 (Item No. 13834).{25262} It is produced from docosahexaenoic acid (DHA; Item No. 90310) by COX-2 in the presence of aspirin via a 17(R)-hydroperoxy DHA (17(R)-HDHA; Item No. 10005099) intermediate and has been found in mouse inflammatory exudates.{25262,45355} 17(R)-RvD3 reduces transmigration of isolated human polymorphonuclear cells (PMNs) and induces efferocytosis of apoptotic PMNs by macrophages.{45355} 17(R)-RvD3 (10 ng/animal) reduces transmigration of neutrophils into the peritoneal cavity, as well as decreases the levels of IL-6 and increases the levels of IL-10 in inflammatory exudate in a mouse model of zymosan-induced peritonitis. It activates GPR32 in a β-arrestin reporter assay and increases phagocytosis to a greater degree in CHO cells overexpressing GPR32 compared to mock-transfected cells. 17(R)-RvD3 increases phagocytosis of etoposide-generated tumor cell debris by monocyte-derived macrophages in H460 human lung carcinoma cells in a concentration-dependent manner.{45356} It also inhibits tumor growth in a mouse model of Lewis lung carcinoma when administered at a dose of 0.6 µg/kg per day.
Brand:CaymanSKU:9002880 - 100 µgAvailable on backorder
17(R)-Resolvin D3 (17(R)-RvD3) is an aspirin-triggered epimer of resolvin D3 (Item No. 13834).{25262} It is produced from docosahexaenoic acid (DHA; Item No. 90310) by COX-2 in the presence of aspirin via a 17(R)-hydroperoxy DHA (17(R)-HDHA; Item No. 10005099) intermediate and has been found in mouse inflammatory exudates.{25262,45355} 17(R)-RvD3 reduces transmigration of isolated human polymorphonuclear cells (PMNs) and induces efferocytosis of apoptotic PMNs by macrophages.{45355} 17(R)-RvD3 (10 ng/animal) reduces transmigration of neutrophils into the peritoneal cavity, as well as decreases the levels of IL-6 and increases the levels of IL-10 in inflammatory exudate in a mouse model of zymosan-induced peritonitis. It activates GPR32 in a β-arrestin reporter assay and increases phagocytosis to a greater degree in CHO cells overexpressing GPR32 compared to mock-transfected cells. 17(R)-RvD3 increases phagocytosis of etoposide-generated tumor cell debris by monocyte-derived macrophages in H460 human lung carcinoma cells in a concentration-dependent manner.{45356} It also inhibits tumor growth in a mouse model of Lewis lung carcinoma when administered at a dose of 0.6 µg/kg per day.
Brand:CaymanSKU:9002880 - 25 µgAvailable on backorder
17(R)-Resolvin D3 (17(R)-RvD3) is an aspirin-triggered epimer of resolvin D3 (Item No. 13834).{25262} It is produced from docosahexaenoic acid (DHA; Item No. 90310) by COX-2 in the presence of aspirin via a 17(R)-hydroperoxy DHA (17(R)-HDHA; Item No. 10005099) intermediate and has been found in mouse inflammatory exudates.{25262,45355} 17(R)-RvD3 reduces transmigration of isolated human polymorphonuclear cells (PMNs) and induces efferocytosis of apoptotic PMNs by macrophages.{45355} 17(R)-RvD3 (10 ng/animal) reduces transmigration of neutrophils into the peritoneal cavity, as well as decreases the levels of IL-6 and increases the levels of IL-10 in inflammatory exudate in a mouse model of zymosan-induced peritonitis. It activates GPR32 in a β-arrestin reporter assay and increases phagocytosis to a greater degree in CHO cells overexpressing GPR32 compared to mock-transfected cells. 17(R)-RvD3 increases phagocytosis of etoposide-generated tumor cell debris by monocyte-derived macrophages in H460 human lung carcinoma cells in a concentration-dependent manner.{45356} It also inhibits tumor growth in a mouse model of Lewis lung carcinoma when administered at a dose of 0.6 µg/kg per day.
Brand:CaymanSKU:9002880 - 50 µgAvailable on backorder
Brand:CaymanSKU:9002881 - 10 µgAvailable on backorder
Brand:CaymanSKU:9002881 - 100 µgAvailable on backorder
Brand:CaymanSKU:9002881 - 25 µgAvailable on backorder
Brand:CaymanSKU:9002881 - 50 µgAvailable on backorder
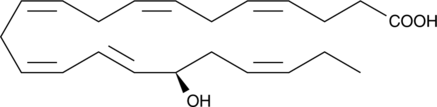
17(S)-HDHA is a hydroxy fatty acid formed from docosahexaenoic acid (DHA; Item No. 90310) by 15-lipoxygenase (15-LO) and is a precursor to 17(S)-resolvins.{13989,46682} 17(S)-HDHA inhibits platelet 12-LO (IC50 = 0.4 μM).{46682} It inhibits TNF-α-induced expression of IL1B in a human glial cell line (IC50 = ~0.5 nM).{13989} 17(S)-HDHA (100 nM) inhibits NOD-, LRR-, and pyrin domain-containing protein 3 (NLRP3) inflammasome formation induced by homocysteine in podocytes.{46683}
Brand:CaymanSKU:10009799 - 100 µgAvailable on backorder
17(S)-HDHA is a hydroxy fatty acid formed from docosahexaenoic acid (DHA; Item No. 90310) by 15-lipoxygenase (15-LO) and is a precursor to 17(S)-resolvins.{13989,46682} 17(S)-HDHA inhibits platelet 12-LO (IC50 = 0.4 μM).{46682} It inhibits TNF-α-induced expression of IL1B in a human glial cell line (IC50 = ~0.5 nM).{13989} 17(S)-HDHA (100 nM) inhibits NOD-, LRR-, and pyrin domain-containing protein 3 (NLRP3) inflammasome formation induced by homocysteine in podocytes.{46683}
Brand:CaymanSKU:10009799 - 25 µgAvailable on backorder
17(S)-HDHA is a hydroxy fatty acid formed from docosahexaenoic acid (DHA; Item No. 90310) by 15-lipoxygenase (15-LO) and is a precursor to 17(S)-resolvins.{13989,46682} 17(S)-HDHA inhibits platelet 12-LO (IC50 = 0.4 μM).{46682} It inhibits TNF-α-induced expression of IL1B in a human glial cell line (IC50 = ~0.5 nM).{13989} 17(S)-HDHA (100 nM) inhibits NOD-, LRR-, and pyrin domain-containing protein 3 (NLRP3) inflammasome formation induced by homocysteine in podocytes.{46683}
Brand:CaymanSKU:-17(S)-HDHA is a hydroxy fatty acid formed from docosahexaenoic acid (DHA; Item No. 90310) by 15-lipoxygenase (15-LO) and is a precursor to 17(S)-resolvins.{13989,46682} 17(S)-HDHA inhibits platelet 12-LO (IC50 = 0.4 μM).{46682} It inhibits TNF-α-induced expression of IL1B in a human glial cell line (IC50 = ~0.5 nM).{13989} 17(S)-HDHA (100 nM) inhibits NOD-, LRR-, and pyrin domain-containing protein 3 (NLRP3) inflammasome formation induced by homocysteine in podocytes.{46683}
Brand:CaymanSKU:10009799 - 250 µgAvailable on backorder
17(S)-HDHA is a hydroxy fatty acid formed from docosahexaenoic acid (DHA; Item No. 90310) by 15-lipoxygenase (15-LO) and is a precursor to 17(S)-resolvins.{13989,46682} 17(S)-HDHA inhibits platelet 12-LO (IC50 = 0.4 μM).{46682} It inhibits TNF-α-induced expression of IL1B in a human glial cell line (IC50 = ~0.5 nM).{13989} 17(S)-HDHA (100 nM) inhibits NOD-, LRR-, and pyrin domain-containing protein 3 (NLRP3) inflammasome formation induced by homocysteine in podocytes.{46683}
Brand:CaymanSKU:10009799 - 50 µgAvailable on backorder
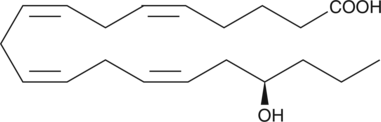
Electrolyte and fluid transport in the kidney are regulated in part by arachidonic acid and its metabolites. Electrolyte and fluid transport in the kidney are regulated in part by arachidonic acid and its metabolites.17-HETE is a cytochrome P450 (CYP450) metabolite of arachidonic acid that has stereospecific effects on sodium transport in the kidney.17(S)-HETE inhibits proximal tubule ATPase activity by as much as 70% at a concentration of 2 µM.{3825}
Brand:CaymanSKU:10011305 - 100 µgAvailable on backorder
Electrolyte and fluid transport in the kidney are regulated in part by arachidonic acid and its metabolites. Electrolyte and fluid transport in the kidney are regulated in part by arachidonic acid and its metabolites.17-HETE is a cytochrome P450 (CYP450) metabolite of arachidonic acid that has stereospecific effects on sodium transport in the kidney.17(S)-HETE inhibits proximal tubule ATPase activity by as much as 70% at a concentration of 2 µM.{3825}
Brand:CaymanSKU:10011305 - 25 µgAvailable on backorder
Electrolyte and fluid transport in the kidney are regulated in part by arachidonic acid and its metabolites. Electrolyte and fluid transport in the kidney are regulated in part by arachidonic acid and its metabolites.17-HETE is a cytochrome P450 (CYP450) metabolite of arachidonic acid that has stereospecific effects on sodium transport in the kidney.17(S)-HETE inhibits proximal tubule ATPase activity by as much as 70% at a concentration of 2 µM.{3825}
Brand:CaymanSKU:10011305 - 50 µgAvailable on backorder
17(S)-HpDHA is a mono-oxygenation product of docosahexaenoic acid in human whole blood, human leukocytes, human glial cells, and mouse brain.{13989} 17(S)-HpDHA is generally reduced to 17(S)-HDHA (Item No. 10009799), a compound that serves as a precursor to 17(S)-resolvins. 17(S)-HDHA has been shown to inhibit TNF-α-induced interleukin-1β expression in human glioma cells and inhibit TNF-α-induced leukocyte trafficking to the murine air pouch.{13989}
Brand:CaymanSKU:-17(S)-HpDHA is a mono-oxygenation product of docosahexaenoic acid in human whole blood, human leukocytes, human glial cells, and mouse brain.{13989} 17(S)-HpDHA is generally reduced to 17(S)-HDHA (Item No. 10009799), a compound that serves as a precursor to 17(S)-resolvins. 17(S)-HDHA has been shown to inhibit TNF-α-induced interleukin-1β expression in human glioma cells and inhibit TNF-α-induced leukocyte trafficking to the murine air pouch.{13989}
Brand:CaymanSKU:-17(S)-HpDHA is a mono-oxygenation product of docosahexaenoic acid in human whole blood, human leukocytes, human glial cells, and mouse brain.{13989} 17(S)-HpDHA is generally reduced to 17(S)-HDHA (Item No. 10009799), a compound that serves as a precursor to 17(S)-resolvins. 17(S)-HDHA has been shown to inhibit TNF-α-induced interleukin-1β expression in human glioma cells and inhibit TNF-α-induced leukocyte trafficking to the murine air pouch.{13989}
Brand:CaymanSKU:-17(S)-HpDHA is a mono-oxygenation product of docosahexaenoic acid in human whole blood, human leukocytes, human glial cells, and mouse brain.{13989} 17(S)-HpDHA is generally reduced to 17(S)-HDHA (Item No. 10009799), a compound that serves as a precursor to 17(S)-resolvins. 17(S)-HDHA has been shown to inhibit TNF-α-induced interleukin-1β expression in human glioma cells and inhibit TNF-α-induced leukocyte trafficking to the murine air pouch.{13989}
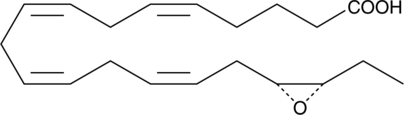
Brand:CaymanSKU:-17R(18S)-EpETE is an oxylipin and a cytochrome P450 metabolite of eicosapentaenoic acid (EPA; Item Nos. 90110 | 90110.1 | 21908).{11951,9825} 17R(18S)-EpETE is an activator of large-conductance calcium-activated potassium (BKCa) channels, increasing the potassium current amplitude by 15-fold in isolated rat cerebral artery vascular smooth muscle cells (VSMCs) at +60 mV when used at a concentration of 50 nM.{9825} It has negative chronotropic effects in isolated neonatal rat cardiomyocytes (NRCMs; EC50 = ~1-2 nM) and prevents calcium-induced increases in the spontaneous beating of NRCMs.{19905,18672}
Brand:CaymanSKU:28630 - 100 µgAvailable on backorder
17R(18S)-EpETE is an oxylipin and a cytochrome P450 metabolite of eicosapentaenoic acid (EPA; Item Nos. 90110 | 90110.1 | 21908).{11951,9825} 17R(18S)-EpETE is an activator of large-conductance calcium-activated potassium (BKCa) channels, increasing the potassium current amplitude by 15-fold in isolated rat cerebral artery vascular smooth muscle cells (VSMCs) at +60 mV when used at a concentration of 50 nM.{9825} It has negative chronotropic effects in isolated neonatal rat cardiomyocytes (NRCMs; EC50 = ~1-2 nM) and prevents calcium-induced increases in the spontaneous beating of NRCMs.{19905,18672}
Brand:CaymanSKU:28630 - 25 µgAvailable on backorder
17R(18S)-EpETE is an oxylipin and a cytochrome P450 metabolite of eicosapentaenoic acid (EPA; Item Nos. 90110 | 90110.1 | 21908).{11951,9825} 17R(18S)-EpETE is an activator of large-conductance calcium-activated potassium (BKCa) channels, increasing the potassium current amplitude by 15-fold in isolated rat cerebral artery vascular smooth muscle cells (VSMCs) at +60 mV when used at a concentration of 50 nM.{9825} It has negative chronotropic effects in isolated neonatal rat cardiomyocytes (NRCMs; EC50 = ~1-2 nM) and prevents calcium-induced increases in the spontaneous beating of NRCMs.{19905,18672}
Brand:CaymanSKU:28630 - 50 µgAvailable on backorder
17R(18S)-EpETE is an oxylipin and a cytochrome P450 metabolite of eicosapentaenoic acid (EPA; Item Nos. 90110 | 90110.1 | 21908).{11951,9825} 17R(18S)-EpETE is an activator of large-conductance calcium-activated potassium (BKCa) channels, increasing the potassium current amplitude by 15-fold in isolated rat cerebral artery vascular smooth muscle cells (VSMCs) at +60 mV when used at a concentration of 50 nM.{9825} It has negative chronotropic effects in isolated neonatal rat cardiomyocytes (NRCMs; EC50 = ~1-2 nM) and prevents calcium-induced increases in the spontaneous beating of NRCMs.{19905,18672}
Brand:CaymanSKU:28630 - 500 µgAvailable on backorder
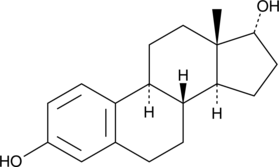
17α-Estradiol is the natural optical isomer of 17β-estradiol (Item No. 10006315), the major estrogen secreted by the premenopausal ovary. 17α-Estradiol is a less active isomer than its counterpart, displaying 100-fold lower estrogenic activity relative to 17β-estradiol.{24968} It can inhibit 5α-reductase, which plays a role in regulating hair growth. 17α -estradiol is reported to activate the MAPK/ERK and PI3K-Akt signaling pathways via activation of the estrogen receptor-X and has been shown to be neuroprotective after an ischemic stroke and oxidative stress and in transgenic mice with Alzheimer’s disease.{24968,13837}
Brand:CaymanSKU:20776 -Available on backorder
17α-Estradiol is the natural optical isomer of 17β-estradiol (Item No. 10006315), the major estrogen secreted by the premenopausal ovary. 17α-Estradiol is a less active isomer than its counterpart, displaying 100-fold lower estrogenic activity relative to 17β-estradiol.{24968} It can inhibit 5α-reductase, which plays a role in regulating hair growth. 17α -estradiol is reported to activate the MAPK/ERK and PI3K-Akt signaling pathways via activation of the estrogen receptor-X and has been shown to be neuroprotective after an ischemic stroke and oxidative stress and in transgenic mice with Alzheimer’s disease.{24968,13837}
Brand:CaymanSKU:20776 -Available on backorder
17α-Estradiol is the natural optical isomer of 17β-estradiol (Item No. 10006315), the major estrogen secreted by the premenopausal ovary. 17α-Estradiol is a less active isomer than its counterpart, displaying 100-fold lower estrogenic activity relative to 17β-estradiol.{24968} It can inhibit 5α-reductase, which plays a role in regulating hair growth. 17α -estradiol is reported to activate the MAPK/ERK and PI3K-Akt signaling pathways via activation of the estrogen receptor-X and has been shown to be neuroprotective after an ischemic stroke and oxidative stress and in transgenic mice with Alzheimer’s disease.{24968,13837}
Brand:CaymanSKU:20776 -Available on backorder
17α-Estradiol is the natural optical isomer of 17β-estradiol (Item No. 10006315), the major estrogen secreted by the premenopausal ovary. 17α-Estradiol is a less active isomer than its counterpart, displaying 100-fold lower estrogenic activity relative to 17β-estradiol.{24968} It can inhibit 5α-reductase, which plays a role in regulating hair growth. 17α -estradiol is reported to activate the MAPK/ERK and PI3K-Akt signaling pathways via activation of the estrogen receptor-X and has been shown to be neuroprotective after an ischemic stroke and oxidative stress and in transgenic mice with Alzheimer’s disease.{24968,13837}
Brand:CaymanSKU:20776 -Available on backorder
17α-hydroxy Pregnenolone is a metabolite of pregnenolone (Item No. 19864) and a precursor in the biosynthesis of dehydroepiandrosterone (DHEA; Item No. 15728).{53115} It is formed from pregnenolone by cytochrome P450 (CYP) isoform CYP17A1 hydroxylase activity and then converted to dehydroepiandrosterone by CYP17A1-mediated 17,20-lyase activity. Plasma levels of 17α-hydroxy pregnenolone are elevated in patients with type II 3β-hydroxysteroid dehydrogenase deficiency, a form of congenital adrenal hyperplasia.{53116}
Brand:CaymanSKU:29181 - 1 gAvailable on backorder
17α-hydroxy Pregnenolone is a metabolite of pregnenolone (Item No. 19864) and a precursor in the biosynthesis of dehydroepiandrosterone (DHEA; Item No. 15728).{53115} It is formed from pregnenolone by cytochrome P450 (CYP) isoform CYP17A1 hydroxylase activity and then converted to dehydroepiandrosterone by CYP17A1-mediated 17,20-lyase activity. Plasma levels of 17α-hydroxy pregnenolone are elevated in patients with type II 3β-hydroxysteroid dehydrogenase deficiency, a form of congenital adrenal hyperplasia.{53116}
Brand:CaymanSKU:29181 - 100 mgAvailable on backorder
17α-hydroxy Pregnenolone is a metabolite of pregnenolone (Item No. 19864) and a precursor in the biosynthesis of dehydroepiandrosterone (DHEA; Item No. 15728).{53115} It is formed from pregnenolone by cytochrome P450 (CYP) isoform CYP17A1 hydroxylase activity and then converted to dehydroepiandrosterone by CYP17A1-mediated 17,20-lyase activity. Plasma levels of 17α-hydroxy pregnenolone are elevated in patients with type II 3β-hydroxysteroid dehydrogenase deficiency, a form of congenital adrenal hyperplasia.{53116}
Brand:CaymanSKU:29181 - 250 mgAvailable on backorder
17α-hydroxy Pregnenolone is a metabolite of pregnenolone (Item No. 19864) and a precursor in the biosynthesis of dehydroepiandrosterone (DHEA; Item No. 15728).{53115} It is formed from pregnenolone by cytochrome P450 (CYP) isoform CYP17A1 hydroxylase activity and then converted to dehydroepiandrosterone by CYP17A1-mediated 17,20-lyase activity. Plasma levels of 17α-hydroxy pregnenolone are elevated in patients with type II 3β-hydroxysteroid dehydrogenase deficiency, a form of congenital adrenal hyperplasia.{53116}
Brand:CaymanSKU:29181 - 500 mgAvailable on backorder
17α,20β-Dihydroxy-4-pregnen-3-one (17α,20β-DHP) is an endogenous, maturation-inducing steroid that stimulates oocyte maturation in females of several teleost species. For example, 1 µg/ml of 17α,20β-DHP applied to Persian sturgeon oocytes has been shown to effectively induce germinal vesicle breakdown, an essential step during oocyte maturation.{26324} Gonadotropin-releasing hormone and the gonadotropins, follicle-stimulating hormone and luteinizing hormone, have been shown to stimulate the production of 17α,20β-DHP either in vivo or in vitro.{26325} 17α,20β-DHP has also been reported to influence spermiation by stimulating milt production when administered at 5 mg/kg to various male teleosts.{26324}
Brand:CaymanSKU:-17α,20β-Dihydroxy-4-pregnen-3-one (17α,20β-DHP) is an endogenous, maturation-inducing steroid that stimulates oocyte maturation in females of several teleost species. For example, 1 µg/ml of 17α,20β-DHP applied to Persian sturgeon oocytes has been shown to effectively induce germinal vesicle breakdown, an essential step during oocyte maturation.{26324} Gonadotropin-releasing hormone and the gonadotropins, follicle-stimulating hormone and luteinizing hormone, have been shown to stimulate the production of 17α,20β-DHP either in vivo or in vitro.{26325} 17α,20β-DHP has also been reported to influence spermiation by stimulating milt production when administered at 5 mg/kg to various male teleosts.{26324}
Brand:CaymanSKU:-