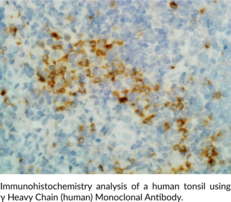
Description
Immunoglobulin G (IgG) is a member of the immunoglobulin superfamily of glycoproteins that plays a central role in the adaptive immune response.{53881,53882} It is produced by B cells and later secreted by plasma cells and is the most abundant circulating antibody in rabbit serum. IgG consists of two identical heavy chains, also known as γ heavy chains, of approximately 50 kDa each and two identical light chains of approximately 25 kDa each.{53881} The heavy chains are linked together by a single disulfide bond to form an Fc region and also combine with the light chains through additional disulfide bonds to form the Fab region, which mediate receptor and antigen binding, respectively. IgG is produced following IgM class-switching in response to infection and is involved in numerous humoral host defense responses, including antibody-dependent cell-mediated cytotoxicity (ADCC), toxin neutralization, and pathogen opsonization.{53881,55170,53883} γ-Heavy chains are truncated and unable to associate with IgG light chains in patients with the rare disease γ heavy chain disease.{53884} Cayman’s γ Heavy Chain (human) Monoclonal antibody can be used for ELISA, immunocytochemistry (ICC), and immunohistochemistry (IHC) applications. The antibody recognizes γ heavy chain from human samples.
Synonyms: IgG Heavy Chain
Immunogen: Human IgG
Formulation: 100 µg of protein A-affinity purified monoclonal antibody
Isotype: IgG
Applications: ELISA, ICC, IHC
Origin:
Stability: 365 days
Application|ELISA||Application|Immunocytochemistry||Application|Immunohistochemistry||Product Type|Antibodies|Monoclonal Antibodies||Research Area|Cardiovascular System|Blood|Serum Proteins||Research Area|Immunology & Inflammation|Adaptive Immunity||Research Area|Infectious Disease