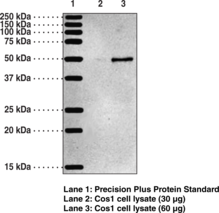
Description
The three dimensional structure of many extracellular proteins is stabilized by the formation of disulphide bonds. Studies suggest that a microsomal enzyme known as protein disulphide isomerase (PDI) is involved in disulphide-bond formation via its oxidase activity and isomerization via its isomerase activity, as well as the reduction of disulphite bonds in proteins.{16242} Studies suggest BiP and PDI work together sequentially to increase oxidation of these proteins.{16273,16274} PDI has also been found to function as a chaperone to prevent the aggregation of unfolded substrates, and serves as a subunit of prolyl 4-hydroxylase and microsomal triglyceride transferase.{16275,16276} PDI is an abundant 55 kDa protein located primarily in the ER, however studies have also proved its presence in the cytosol.{16272} PDI has the ability to reside in the ER permanently due to the highly conserved KDEL sequence at its carboxy-terminus.{16277} It uses carboxy-terminal KDEL as a retention signal, and this appears to be sufficient to reduce the secretion of proteins from the ER. This retention is reported to be mediated by a KDEL receptor.{16278}
Synonyms: Protein Disulphide Isomerase
Immunogen: Synthetic peptide from rat PDI conjugated to KLH
Formulation:
Isotype:
Applications: WB, IP, ICC, IHC
Origin: Animal/Rabbit
Stability: 365 days
Application|Immunocytochemistry||Application|Immunohistochemistry||Application|Immunoprecipitation||Application|Western Blot||Product Type|Antibodies|Polyclonal Antibodies