Description
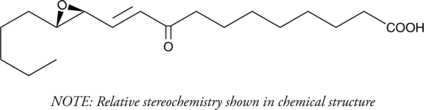
A biologically active peroxidation product of linoleic acid; activates an ARE in neuronal cells and induces the expression of ARE-regulated cytoprotective genes; also stimulates the synthesis of aldosterone and corticosterone in adrenal cells when supplied at 1-5 μM
Formal name: 9-oxo-11-(3-pentyl-2-oxiranyl)-10E-undecenoic acid
Synonyms: 12,13-epoxy-9-keto-10(trans)-Octadecenoic Acid
Molecular weight: 310.4
CAS: 478931-82-7
Purity: ≥98%
Formulation: A solution in ethanol
Product Type|Biochemicals|Lipids|Octadecanoids||Product Type|Biochemicals|Ox Stress Reagents|Antioxidants||Research Area|Lipid Biochemistry|Reactive O2/N2 Pathways||Research Area|Oxidative Stress & Reactive Species|Lipid Peroxidation