Description
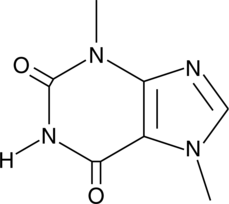
A methylxnthine alkaloid with diverse biological activities; derivative of caffeine; adenosine A1 receptor antagonist (IC50s = 200-280 μM in radioligand binding assays using rat brain membranes); increases AMPK phosphorylation and inhibits adipocyte differentiation, ERK and JNK phosphorylation, and IL-6 and TNF-α production in 3T3-L1 preadipocytes cultured in differentiation medium at 150 μg/ml; inhibits decreases in renal cortex SIRT1 activity and increases in NADPH oxidase-dependent ROS production, as well as reduces kidney hypertrophy and albuminuria in a spontaneously hypertensive rat model of streptozotocin-induced diabetes at 5 mg/kg per day; toxic to dogs (LD50 = 250-500 mg/kg)
Formal name: 3,7-dihydro-3,7-dimethyl-1H-purine-2,6-dione
Synonyms: Diurobromine|NSC 5039|SC-15090
Molecular weight: 180.2
CAS: 83-67-0
Purity: ≥98%
Formulation: A crystalline solid
Product Type|Biochemicals|Natural Products|Alkaloids||Product Type|Biochemicals|Ox Stress Reagents|Antioxidants||Product Type|Biochemicals|Receptor Pharmacology|Antagonists||Research Area|Cardiovascular System|Kidney & Renal Disease|Diabetic Nephropathy||Research Area|Endocrinology & Metabolism|Metabolic Diseases|Diabetes||Research Area|Immunology & Inflammation||Research Area|Neuroscience||Research Area|Oxidative Stress & Reactive Species|Antioxidant Activity||Research Area|Oxidative Stress & Reactive Species|Reactive Oxygen||Research Area|Toxicology