Description
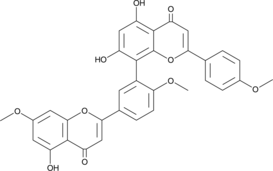
A biflavonoid with diverse biological activities; reduces Aβ42-induced cytotoxicity in PC12 cells (EC50 = 9.84 μM); decreases methylglyoxal-induced insulin secretion, production of ROS, cardiolipin peroxidation, and cytotoxicity in RIN-m5F pancreatic β-cells from 0.1-10 μM; inhibits P-gp (IC50 = 53.42 μM) and increases cellular toxicity of paraquat and paclitaxel in MDR1-MDCKII cells; inhibits RANKL-induced mRNA expression of the osteoclast-specific genes CTSK, TRAP, and MMP-9, activation of NF-κB, and osteoclastogenesis in a mouse model of LPS-induced bone loss,
Formal name: 5,7-dihydroxy-8-[5-(5-hydroxy-7-methoxy-4-oxo-4H-1-benzopyran-2-yl)-2-methoxyphenyl]-2-(4-methoxyphenyl)-4H-1-benzopyran-4-one
Synonyms: NSC 45108
Molecular weight: 580.5
CAS: 521-34-6
Purity: ≥98%
Formulation: A solid
Product Type|Biochemicals|Natural Products|Flavonoids||Product Type|Biochemicals|Transporter & Exchanger Modulators||Research Area|Endocrinology & Metabolism|Bone Growth & Remodeling||Research Area|Endocrinology & Metabolism|Metabolic Diseases|Diabetes||Research Area|Neuroscience|Neuroprotection||Research Area|Oxidative Stress & Reactive Species|Antioxidant Activity||Research Area|Oxidative Stress & Reactive Species|Lipid Peroxidation||Research Area|Oxidative Stress & Reactive Species|Reactive Oxygen||Research Area|Toxicology|Drug Metabolism