Description
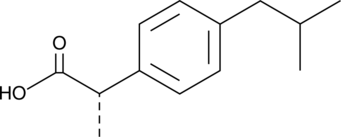
An enantiomer of ibuprofen that more potently inhibits COX activity, thromboxane formation, and platelet aggregation than the (R)-form; also inhibits activation of NF-κB more effectively than (R)-ibuprofen (IC50s = 62 and 122 µM, respectively)
Formal name: (αS)-α-methyl-4-(2-methylpropyl)-benzeneacetic acid
Synonyms: (+)-Ibuprofen|Dexibuprofen
Molecular weight: 206.3
CAS: 51146-56-6
Purity: ≥98%
Formulation: A crystalline solid
Product Type|Biochemicals|Small Molecule Inhibitors|Cyclooxygenases||Research Area|Immunology & Inflammation|Inflammatory Lipid Mediators|Leukotrienes||Research Area|Immunology & Inflammation|Inflammatory Lipid Mediators|Prostaglandins||Research Area|Immunology & Inflammation|Innate Immunity||Research Area|Lipid Biochemistry|Cyclooxygenase Pathway||Research Area|Lipid Biochemistry|Lipoxygenase Pathways