Description
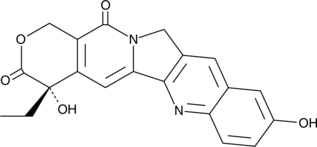
An inhibitor of topoisomerase I that demonstrates less toxicity than its parent compound; demonstrates strong anti-tumor activity against a wide range of experimental tumors including L1210 leukemia cells (IC50 = 1.15 μM)
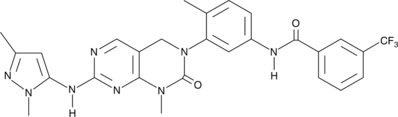
Formal name: (4S)-4-ethyl-4,9-dihydroxy-1H-pyrano[3′,4′:6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione
Synonyms: ChEMBL 273862|NSC 107124
Molecular weight: 364.4
CAS: 19685-09-7
Purity: ≥98%
Formulation: A crystalline solid
Product Type|Biochemicals|Natural Products||Product Type|Biochemicals|Small Molecule Inhibitors|MMPs||Product Type|Biochemicals|Small Molecule Inhibitors|Topoisomerases||Research Area|Cancer|Cell Cycle|G2/M||Research Area|Cancer|DNA Damage and Repair|Topoisomerase