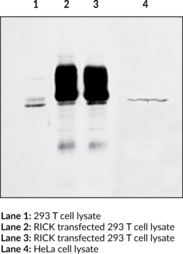
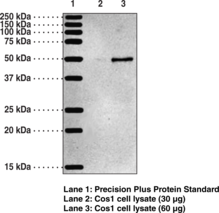
Description
Apoptosis is mediated by death domain (DD) and/or caspase recruitment domain (CARD) containing molecules and a caspase family of proteases. The DD-containing serine/threonine kinase RIP regulates Fas-induced apoptosis. A novel CARD-containing serine/threonine kinase that regulates apoptosis was identified and designated RICK for RIP-like interacting CLARP kinase.{7167} RICK contains an N-terminal kinase catalytic domain and a C-terminal CARD domain. The RICK kinase domain has high sequence homology to that of RIP. Overexpression of RICK promotes the activation of caspase-8 and Fas-induced apoptosis. RICK represents a novel kinase that regulates Fas-induced apoptosis. The mRNA for RICK is expressed in multiple human tissues.{7167} RICK interacts with several Nod and T-cell receptor proteins and thereby confers signal transduction for innate and adaptive immune system responses.{12843}
Synonyms: CARDIAK|Rip2|Ripk2
Immunogen: Synthetic peptide from the N-terminal region of human RICK
Formulation: 500 µl of Peptide affinity-purified polyclonal antibody
Isotype:
Applications: WB
Origin: Animal/Rabbit
Stability: 365 days
Application|Western Blot||Product Type|Antibodies|Polyclonal Antibodies||Research Area|Cancer|Cell Death|Apoptosis||Research Area|Cancer|Cell Signaling||Research Area|Cell Biology|Cell Death|Apoptosis||Research Area|Cell Biology|Proteolysis|Cytosolic & Secreted Proteases||Research Area|Immunology & Inflammation|Adaptive Immunity||Research Area|Immunology & Inflammation|Innate Immunity