Description
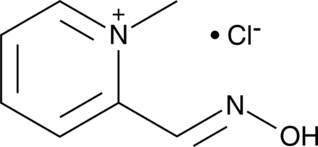
Reactivates AChE that has been deactivated by binding of organophosphates to its esteratic site; binds to the anionic site of AChE and displaces the phosphate from the esteratic site through formation of phosphate-pralidoxime conjugates; reactivates paraoxon- and diisopropyl fluorophosphate-inactivated human RBC AChE with IC50 shifts of 0.3 and 0.8 nM per μM of pralidoxime, respectively; at a concentration of 10 μM, reactivates human RBC AChE that has been inactivated by chlorpyrifos, diazinon, and malathion by 17, 61, and 36%, respectively; binds to sarin-bound hAChE (Kd = 25.72 μM) and inhibits sarin-induced AChE deactivation (IC50 = 1.21 mM) in hemoglobin-free erythrocyte ghosts
Formal name: 2-[(hydroxyimino)methyl]-1-methyl-pyridinium, monochloride
Synonyms: 2-PAM
Molecular weight: 172.6
CAS: 51-15-0
Purity: ≥95%
Formulation: A crystalline solid
Product Type|Biochemicals|Small Molecule Activators||Research Area|Neuroscience||Research Area|Toxicology|Environmental