Description
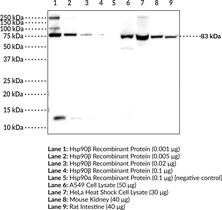
Heat shock protein 90 β (Hsp90β) is the constitutively active cytosolic isoform of Hsp90 that is encoded by HSP90AB in humans.{17930} Hsp90 is a multidomain protein that functions as a molecular chaperone to assist in folding and activation of nascent peptides, refolding unfolded or misfolded proteins, and preventing protein aggregation.{15502} C-terminal dimerization of Hsp90, coupled with ATPase molecular clamp activity induces a conformational change in the N-terminal nucleotide binding domain that facilitates substrate binding and initiates the chaperone cycle.{17932} Hsp90 interacts with many co-chaperones during its chaperone cycle including p23 and Sba1, which help recruit substrates to the Hsp90 complex, Hsp70 (Item Nos. 22739 | 23002), which loads nascent polypeptides onto the Hsp90 dimer, and the ATPase activator Aha1 that promotes ATP hydrolysis and substrate release.{17931,41851} Hsp90 is overexpressed in cancer cells and stabilizes client proteins that promote oncogenesis, including transcription factors, signaling proteins, and kinases.{17930,41851} Hsp90 also decreases α-synuclein fibril formation and toxicity as well as Q35 aggregation in in vitro models of Parkinson’s and Huntington’s disease, respectively, implying a role in neurodegenerative disease.{41852} Cayman’s Hsp90β Polyclonal Antibody can be used for Western blot, ELISA, IHC, and IF applications. This antibody recognizes Hsp90β at 83 kDa from human, mouse, and rat samples.
Synonyms: Heat Shock 84 kDa|Heat Shock Protein 90β|Hsp 84
Immunogen: Recombinant full-length human Hsp90β protein
Formulation: 500 µl of protein affinity-purified antibody
Isotype:
Applications:
Origin:
Stability: 365 days
Application|ELISA||Application|Immunofluorescence||Application|Western Blot||Product Type|Antibodies|Polyclonal Antibodies||Research Area|Cancer||Research Area|Neuroscience|Neurodegenerative Disorders|Huntington’s Disease||Research Area|Neuroscience|Neurodegenerative Disorders|Parkinson’s Disease