Description
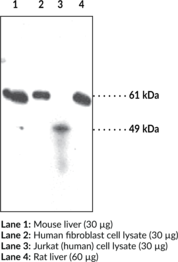
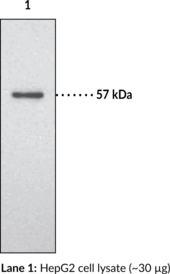
Hsp70/Hsp90 organizing protein (HOP), also known as stress-induced phosphoprotein 1 (STI1 or STIP1), is a co-chaperone that reversibly forms a complex with heat shock protein 70 (Hsp70) and Hsp90 during the Hsp90 chaperone cycle to facilitate the transfer of client proteins from Hsp70 to Hsp90. It contains three tetratricopeptide repeat domains (TPR1, TPR2A, and TPR2B) that act as binding regions, with TPR1 and TPR2B binding to Hsp70 (Item Nos. 22739 | 23002) and TPR2A binding to Hsp90 (Item Nos. 22734 | 22735).{39121} HOP is both a nuclear and cytoplasmic protein, capable of travel between both.{39122} HOP has also been found to interact with several other proteins and complexes, including but not limited to Hsc70 (Item No. 22737), PACRG, METTL21B, FLCN, FNIP1, and FNIP2.{39123,39124,39125,39126,39127} Cayman’s HOP (STI1) Polyclonal Antibody detects HOP (STI1) at 63 kDa.
Synonyms: HOP|Hsc70/Hsp90 Organizing Protein|Renal Carcinoma Antigen NY-REN-11|Stress-Induced Phosphoprotein 1|Transformation-sensitive Protein IEF SSP 3521
Immunogen: Human recombinant HOP
Formulation: 100 µg of protein A-purified polyclonal antibody
Isotype:
Applications: ELISA, IF, and WB
Origin:
Stability: 365 days
Application|ELISA||Application|Immunofluorescence||Application|Western Blot||Product Type|Antibodies|Polyclonal Antibodies||Research Area|Cell Biology|Cellular Chaperones