Description
Hsp90 is crucial to cellular signaling by its regulation of the folding, activity, and stability of a wide range of client proteins. These client protein complexes may also contain one or more co-chaperones.{15726} One class of Hsp90-binding co-chaperone is composed of proteins with a characteristic tetratricopeptide repeat (TPR) domain that forms an Hsp90 binding site. Among the TRP co-chaperones of Hsp90 are Hop/Sti1, protein phosphatase PP5, and members of both the FK506- and cyclosporin A-binding families of immunophilins.{15727} FK506-binding protein 51 (FKBP51) and FKBP52 are large molecular weight immunophilins that are part of the mature glucocorticoid receptor (GR) heterocomplex.{15728} The N-terminal domain of each protein binds FK506 and has peptidyl-prolyl isomerase (PPlase) activity that converts prolyl peptide bonds within target proteins from cis- to trans- proline. The C-terminal domains contain the TRP repeats involved in protein-protein interactions with Hsp40.{15729} Although FKBP52 and FKBP51 share ~75% sequence similarity, they affect hormone binding by the glucocorticoid receptor in opposing manners and have different Hsp90-binding characteristics.{15728,15730} Also, whereas FKBP51 typically has a role with the progesterone receptor, FKBP52 has been found to be linked to the progesterone, androgen, and glucocorticoid receptors.{15730}
Synonyms: FK-506 Binding Protein 52
Immunogen: synthetic peptide corresponding to the residues of human FKBP52
Formulation: Protein G-purified IgG at a concentration of 1 mg/ml in PBS buffer, containing 0.09% sodium azide and 50% glycerol
Isotype: IgG1
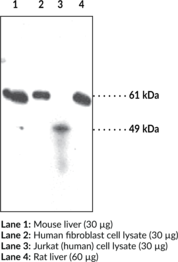
Applications: IHC (paraffin embeded sections), IP, and WB
Origin: Animal/Mouse
Stability: 365 days
Application|Immunohistochemistry||Application|Immunoprecipitation||Application|Western Blot||Product Type|Antibodies|Monoclonal Antibodies||Research Area|Cell Biology|Cellular Chaperones