Description
A small molecule that at 1-25 µM improves ER protein-folding ability, stimulates the expression of multiple chaperone proteins, and induces phosphorylation of eIF2α to reduce protein synthesis; exerts antidiabetic activity in both ob/ob and diet-induced obese mice at 150 mg/kg, improving insulin sensitivity and glucose homeostasis, as well as protecting pancreatic β cells against ER stress
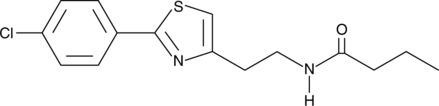
Formal name: N-[2-[2-(4-chlorophenyl)-4-thiazolyl]ethyl]-butanamide
Synonyms:
Molecular weight: 308.8
CAS: 932986-18-0
Purity: ≥98%
Formulation: A crystalline solid
Product Type|Biochemicals||Research Area|Cell Biology|Cellular Chaperones||Research Area|Cell Biology|Endomembrane System & Vesicular Trafficking|ER Protein Folding||Research Area|Endocrinology & Metabolism|Metabolic Diseases|Diabetes