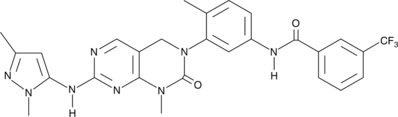
Description
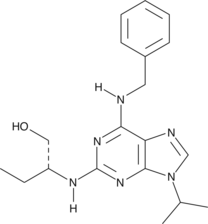
Arachidonamide is an analog of AEA that lacks the hydroxyethyl moiety. It is hydrolyzed by FAAH more effectively than AEA but exhibits significantly weaker binding to the human CB1 receptor with a Ki value of 9.6 µM. Arachidonamide and AEA exhibit similar binding and translocation into cells via the AEA transporter. Arachidonamide inhibits [3H]-AEA uptake into human astrocytoma cells with an IC50 value of 9 µM and inhibits rat glial gap junction cell-cell communication by 90% at a concentration of 20 µM.
Formal name: 5Z,8Z,11Z,14Z-eicosatetraenamide
Synonyms: Arachidonamide|Arachidonic Acid amide
Molecular weight: 303.5
CAS: 85146-53-8
Purity: >98%
Formulation: A solution in methyl acetate
Product Type|Biochemicals|Lipids|Fatty Amides||Product Type|Biochemicals|Receptor Pharmacology||Research Area|Lipid Biochemistry|Endocannabinoid/Endocannabinoid-like||Research Area|Lipid Biochemistry|Lipid Transport||Research Area|Neuroscience|Cannabinoid Research|CB1 & CB2 Receptors||Research Area|Neuroscience|Cannabinoid Research|Endocannabinoids
![Arachidonamide is an analog of AEA that lacks the hydroxyethyl moiety. It is hydrolyzed by FAAH more effectively than AEA but exhibits significantly weaker binding to the human CB1 receptor with a Ki value of 9.6 µM. Arachidonamide and AEA exhibit similar binding and translocation into cells via the AEA transporter. Arachidonamide inhibits [3H]-AEA uptake into human astrocytoma cells with an IC50 value of 9 µM and inhibits rat glial gap junction cell-cell communication by 90% at a concentration of 20 µM.](https://interpriseusa.com/wp-content/uploads/2021/06/10007295.png)