Description
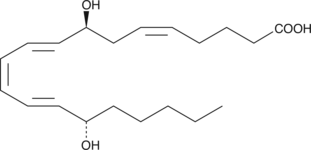
8(S),15(S)-DiHETE is formed when 15(S)-HETE is subjected to further oxidation by 15-LO.{2161} It causes eosinophil chemotaxis with an ED50 value of 1.5 µM but is not chemotactic for neutrophils. 8(S),15(S)-DiHETE antagonizes the hyperalgesic activity of 8(R),15(S)-DiHETE and LTB4 in the rat hind paw pain model.{338}
Formal name: 8S,15S-dihydroxy-5Z,9E,11Z,13E-eicosatetraenoic acid
Synonyms:
Molecular weight: 336.5
CAS: 80234-65-7
Purity: ≥98%
Formulation: A solution in ethanol