Description
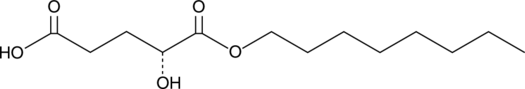
A cell-permeable derivative of the D-isomer of 2-HG; used to examine the contribution of D-2HG to the oxidative mitochondrial processes of IDH1-mutated cancer cells
Formal name: 2R-hydroxy-pentanedioic acid, 1-octyl ester
Synonyms: (2R)-Octyl-2-HG
Molecular weight: 260.3
CAS: 1391194-67-4
Purity: ≥95%
Formulation: A crystalline solid
Product Type|Biochemicals||Research Area|Cancer|Metabolism||Research Area|Neuroscience