Description
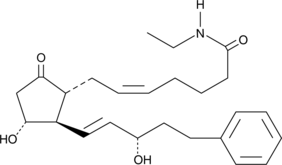
Derived from 17-phenyl trinor PGE2, a synthetic analog of PGE2 that acts as an agonist of EP1 and EP3 in mice (Ki = 14 and 3.7 nM, respectively) and EP1, EP3, and EP4 in rats (Ki = 25, 4.3, and 54 nM, respectively); the ethyl amide group increases lipid solubility, lowering the effective concentration
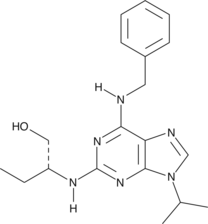
Formal name: N-ethyl-9-oxo-11α,15S-dihydroxy-17-phenyl-18,19,20-trinor-prosta-5Z,13E-dien-1-amide
Synonyms: 17-phenyl trinor PGE2 ethyl amide
Molecular weight: 413.6
CAS: 1219032-20-8
Purity: ≥98%
Formulation: A solution in ethanol
Product Type|Biochemicals|Lipids|Prostaglandins||Product Type|Biochemicals|Receptor Pharmacology|Agonists||Research Area|Endocrinology & Metabolism|Reproductive Biology||Research Area|Lipid Biochemistry|Cyclooxygenase Pathway