Description
Less potent than AEA (Item No. 90050) at the CB1 receptor (Ki = 600 versus 90 nM); inhibits FAAH
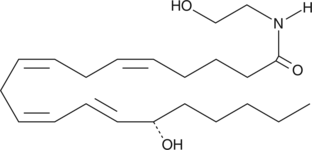
Formal name: 15(S)-hydroxy-N-(2-hydroxyethyl)-5Z,8Z,11Z,13E-eicosatetraenamide
Synonyms: 15(S)-HAEA
Molecular weight: 363.5
CAS: 161744-53-2
Purity: ≥98%
Formulation: A solution in ethanol
Product Type|Biochemicals|Lipids|Fatty Amides||Product Type|Biochemicals|Receptor Pharmacology|Agonists||Product Type|Biochemicals|Small Molecule Inhibitors|Fatty Acid Metabolism||Research Area|Lipid Biochemistry|Endocannabinoid/Endocannabinoid-like||Research Area|Lipid Biochemistry|Lipoxygenase Pathways||Research Area|Neuroscience|Cannabinoid Research|CB1 & CB2 Receptors||Research Area|Neuroscience|Cannabinoid Research|Endocannabinoids