Cayman
Showing 4801–4950 of 45550 results
-
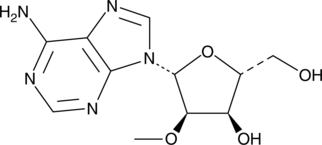
2′-O-Methyladenosine is an analog of adenosine used to prepare nucleoside derivatives as inhibitors of viral RNA translation and replication.{27606}
Brand:CaymanSKU:-Out of stock
2′-O-Methyladenosine is an analog of adenosine used to prepare nucleoside derivatives as inhibitors of viral RNA translation and replication.{27606}
Brand:CaymanSKU:-Out of stock
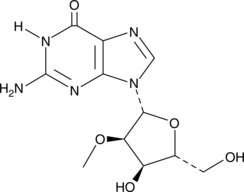
2′-O-Methylguanosine is a modified nucleoside that is produced in tRNAs by the action of tRNA guanosine-2’-O-methyltransferase, using S-adenosyl-L-methionine (Item Nos. 16376 | 13956) as a substrate.{33197,33200} Through its interaction with other modified nucleosides, 2′-O-methylguanosine is thought to stabilize the structure of the tRNA.{33199} 2′-O-Methylguanosine can also be found in rRNA.{33195,33198} Normal and modified nucleosides, including 2′-O-methylguanosine, have been shown to be secreted by a natural suppressor cell line and induce apoptosis in K562/Molt4 target cells.{33196}
Brand:CaymanSKU:21039 -Out of stock
2′-O-Methylguanosine is a modified nucleoside that is produced in tRNAs by the action of tRNA guanosine-2’-O-methyltransferase, using S-adenosyl-L-methionine (Item Nos. 16376 | 13956) as a substrate.{33197,33200} Through its interaction with other modified nucleosides, 2′-O-methylguanosine is thought to stabilize the structure of the tRNA.{33199} 2′-O-Methylguanosine can also be found in rRNA.{33195,33198} Normal and modified nucleosides, including 2′-O-methylguanosine, have been shown to be secreted by a natural suppressor cell line and induce apoptosis in K562/Molt4 target cells.{33196}
Brand:CaymanSKU:21039 -Out of stock
2′-O-Methylguanosine is a modified nucleoside that is produced in tRNAs by the action of tRNA guanosine-2’-O-methyltransferase, using S-adenosyl-L-methionine (Item Nos. 16376 | 13956) as a substrate.{33197,33200} Through its interaction with other modified nucleosides, 2′-O-methylguanosine is thought to stabilize the structure of the tRNA.{33199} 2′-O-Methylguanosine can also be found in rRNA.{33195,33198} Normal and modified nucleosides, including 2′-O-methylguanosine, have been shown to be secreted by a natural suppressor cell line and induce apoptosis in K562/Molt4 target cells.{33196}
Brand:CaymanSKU:21039 -Out of stock
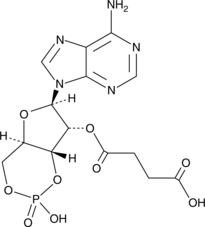
2’-O-Monosuccinyladenosine-3’,5’-cyclic monophosphate is an immunogenic derivative of cAMP that has been used to generate anti-cAMP antisera and antibodies.{54121,54120}
Brand:CaymanSKU:30535 - 10 mgAvailable on backorder
2’-O-Monosuccinyladenosine-3’,5’-cyclic monophosphate is an immunogenic derivative of cAMP that has been used to generate anti-cAMP antisera and antibodies.{54121,54120}
Brand:CaymanSKU:30535 - 25 mgAvailable on backorder
2’-O-Monosuccinyladenosine-3’,5’-cyclic monophosphate is an immunogenic derivative of cAMP that has been used to generate anti-cAMP antisera and antibodies.{54121,54120}
Brand:CaymanSKU:30535 - 5 mgAvailable on backorder
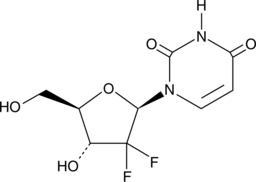
2′,2′-Difluoro-2′-deoxyuridine is an active metabolite of the anticancer nucleoside analog gemcitabine (Item No. 11690).{52668} It is formed by deamination of gemcitabine by cytidine deaminase in the liver. 2′,2′-Difluoro-2′-deoxyuridine is cytotoxic to HepG2 and A549 cancer cells (IC50s = 3.13 and 3.92 µM, respectively). It enhances radiation-induced cell death of ECV304 and NCI H292 cells when used at concentrations ranging from 10 to 100 µM.{52669}
Brand:CaymanSKU:11689 - 1 mgAvailable on backorder
2′,2′-Difluoro-2′-deoxyuridine is an active metabolite of the anticancer nucleoside analog gemcitabine (Item No. 11690).{52668} It is formed by deamination of gemcitabine by cytidine deaminase in the liver. 2′,2′-Difluoro-2′-deoxyuridine is cytotoxic to HepG2 and A549 cancer cells (IC50s = 3.13 and 3.92 µM, respectively). It enhances radiation-induced cell death of ECV304 and NCI H292 cells when used at concentrations ranging from 10 to 100 µM.{52669}
Brand:CaymanSKU:11689 - 10 mgAvailable on backorder
2′,2′-Difluoro-2′-deoxyuridine is an active metabolite of the anticancer nucleoside analog gemcitabine (Item No. 11690).{52668} It is formed by deamination of gemcitabine by cytidine deaminase in the liver. 2′,2′-Difluoro-2′-deoxyuridine is cytotoxic to HepG2 and A549 cancer cells (IC50s = 3.13 and 3.92 µM, respectively). It enhances radiation-induced cell death of ECV304 and NCI H292 cells when used at concentrations ranging from 10 to 100 µM.{52669}
Brand:CaymanSKU:11689 - 25 mgAvailable on backorder
2′,2′-Difluoro-2′-deoxyuridine is an active metabolite of the anticancer nucleoside analog gemcitabine (Item No. 11690).{52668} It is formed by deamination of gemcitabine by cytidine deaminase in the liver. 2′,2′-Difluoro-2′-deoxyuridine is cytotoxic to HepG2 and A549 cancer cells (IC50s = 3.13 and 3.92 µM, respectively). It enhances radiation-induced cell death of ECV304 and NCI H292 cells when used at concentrations ranging from 10 to 100 µM.{52669}
Brand:CaymanSKU:11689 - 5 mgAvailable on backorder
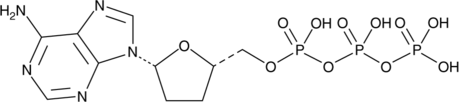
2′,3′-Dideoxyadenosine 5′-triphosphate (ddATP) is an in vitro inhibitor of reverse transcriptases from retroviruses, including HIV-1 and visna (Kis = 20 and 37 nM, respectively).{28262,28268,28264} It also blocks, in vitro, mammalian and bacterial DNA polymerases.{28265,28263} ddATP, produced intracellularly by the phosphorylation of exogenously supplied 2’,3’-dideoxyadenosine, competes with dATP, resulting in chain termination.{28265,28263} Because of this activity, dideoxynucleoside 5’-triphosphates, including ddATP, are used to terminate chain extension produced by the Taq polymerase used in polymerase chain reactions.{28266} It is also an antagonist of the purinergic receptor P2X2/3 (pIC50 = 6.5).{28267}
Brand:CaymanSKU:-Available on backorder
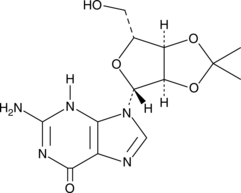
2′,3′-O-Isopropylideneguanosine is an alkylated guanosine building block.{56014,56015} It has been used in the synthesis of ordered honeycomb microporous films and mRNA cap analogs.
Brand:CaymanSKU:30476 - 1 gAvailable on backorder
2′,3′-O-Isopropylideneguanosine is an alkylated guanosine building block.{56014,56015} It has been used in the synthesis of ordered honeycomb microporous films and mRNA cap analogs.
Brand:CaymanSKU:30476 - 500 mgAvailable on backorder
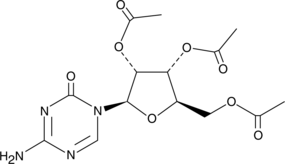
5-Azacytidine is an inhibitor of DNA methyltransferase, potentially serving to reverse epigenetic changes.{17258} It reduces hypermethylation associated with certain diseases, including myelodysplastic syndromes and cancer.{18356,18373,17257} 2’,3’,5’-triacetyl-5-Azacytidine is a prodrug form of 5-azacytidine that may be rapidly absorbed orally without formation of major metabolites in the gastrointestinal tract.
Brand:CaymanSKU:-5-Azacytidine is an inhibitor of DNA methyltransferase, potentially serving to reverse epigenetic changes.{17258} It reduces hypermethylation associated with certain diseases, including myelodysplastic syndromes and cancer.{18356,18373,17257} 2’,3’,5’-triacetyl-5-Azacytidine is a prodrug form of 5-azacytidine that may be rapidly absorbed orally without formation of major metabolites in the gastrointestinal tract.
Brand:CaymanSKU:-5-Azacytidine is an inhibitor of DNA methyltransferase, potentially serving to reverse epigenetic changes.{17258} It reduces hypermethylation associated with certain diseases, including myelodysplastic syndromes and cancer.{18356,18373,17257} 2’,3’,5’-triacetyl-5-Azacytidine is a prodrug form of 5-azacytidine that may be rapidly absorbed orally without formation of major metabolites in the gastrointestinal tract.
Brand:CaymanSKU:-5-Azacytidine is an inhibitor of DNA methyltransferase, potentially serving to reverse epigenetic changes.{17258} It reduces hypermethylation associated with certain diseases, including myelodysplastic syndromes and cancer.{18356,18373,17257} 2’,3’,5’-triacetyl-5-Azacytidine is a prodrug form of 5-azacytidine that may be rapidly absorbed orally without formation of major metabolites in the gastrointestinal tract.
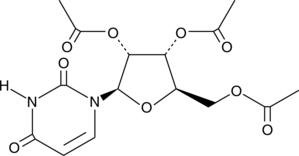
Brand:CaymanSKU:-2’,3’,5’-Triacetyluridine is a prodrug of uridine (Item No. 20300).{46334} It is more lipid soluble than uridine and resistant to degradation by uridine phosphorylase. It is cleaved by plasma esterases in vivo to release uridine. 2’,3’,5’-Triacetyluridine (6% in the diet) decreases neurodegeneration in the piriform cortex and striatum, as well as reduces the amount of huntingtin-positive aggregates and increases BDNF protein levels in the piriform cortex in a transgenic mouse model of Huntington’s disease.{46335} It also improves rotarod performance and increases survival in transgenic mouse models of Huntington’s disease. 2’,3’,5’-Triacetyluridine reverses toxicity and increases survival in a mouse model of dihydropyrimidine dehydrogenase (DPD) deficiency-induced 5-fluorouracil (5-FU; Item No. 14416) overdose when used at a concentration of 2,000 mg/kg three times per day beginning within 24 hours of 5-FU administration.{46336} Formulations containing 2’,3’,5’-triacetyluridine have been used in the treatment of hereditary orotic aciduria and of overdose or life-threatening toxicity due to flurouracil or capecitabine.
Brand:CaymanSKU:27445 - 10 gAvailable on backorder
2’,3’,5’-Triacetyluridine is a prodrug of uridine (Item No. 20300).{46334} It is more lipid soluble than uridine and resistant to degradation by uridine phosphorylase. It is cleaved by plasma esterases in vivo to release uridine. 2’,3’,5’-Triacetyluridine (6% in the diet) decreases neurodegeneration in the piriform cortex and striatum, as well as reduces the amount of huntingtin-positive aggregates and increases BDNF protein levels in the piriform cortex in a transgenic mouse model of Huntington’s disease.{46335} It also improves rotarod performance and increases survival in transgenic mouse models of Huntington’s disease. 2’,3’,5’-Triacetyluridine reverses toxicity and increases survival in a mouse model of dihydropyrimidine dehydrogenase (DPD) deficiency-induced 5-fluorouracil (5-FU; Item No. 14416) overdose when used at a concentration of 2,000 mg/kg three times per day beginning within 24 hours of 5-FU administration.{46336} Formulations containing 2’,3’,5’-triacetyluridine have been used in the treatment of hereditary orotic aciduria and of overdose or life-threatening toxicity due to flurouracil or capecitabine.
Brand:CaymanSKU:27445 - 25 gAvailable on backorder
2’,3’,5’-Triacetyluridine is a prodrug of uridine (Item No. 20300).{46334} It is more lipid soluble than uridine and resistant to degradation by uridine phosphorylase. It is cleaved by plasma esterases in vivo to release uridine. 2’,3’,5’-Triacetyluridine (6% in the diet) decreases neurodegeneration in the piriform cortex and striatum, as well as reduces the amount of huntingtin-positive aggregates and increases BDNF protein levels in the piriform cortex in a transgenic mouse model of Huntington’s disease.{46335} It also improves rotarod performance and increases survival in transgenic mouse models of Huntington’s disease. 2’,3’,5’-Triacetyluridine reverses toxicity and increases survival in a mouse model of dihydropyrimidine dehydrogenase (DPD) deficiency-induced 5-fluorouracil (5-FU; Item No. 14416) overdose when used at a concentration of 2,000 mg/kg three times per day beginning within 24 hours of 5-FU administration.{46336} Formulations containing 2’,3’,5’-triacetyluridine have been used in the treatment of hereditary orotic aciduria and of overdose or life-threatening toxicity due to flurouracil or capecitabine.
Brand:CaymanSKU:27445 - 5 gAvailable on backorder
2’,3’,5’-Triacetyluridine is a prodrug of uridine (Item No. 20300).{46334} It is more lipid soluble than uridine and resistant to degradation by uridine phosphorylase. It is cleaved by plasma esterases in vivo to release uridine. 2’,3’,5’-Triacetyluridine (6% in the diet) decreases neurodegeneration in the piriform cortex and striatum, as well as reduces the amount of huntingtin-positive aggregates and increases BDNF protein levels in the piriform cortex in a transgenic mouse model of Huntington’s disease.{46335} It also improves rotarod performance and increases survival in transgenic mouse models of Huntington’s disease. 2’,3’,5’-Triacetyluridine reverses toxicity and increases survival in a mouse model of dihydropyrimidine dehydrogenase (DPD) deficiency-induced 5-fluorouracil (5-FU; Item No. 14416) overdose when used at a concentration of 2,000 mg/kg three times per day beginning within 24 hours of 5-FU administration.{46336} Formulations containing 2’,3’,5’-triacetyluridine have been used in the treatment of hereditary orotic aciduria and of overdose or life-threatening toxicity due to flurouracil or capecitabine.
Brand:CaymanSKU:27445 - 50 gAvailable on backorder
2′,5′-dideoxy Adenosine is a nucleoside analog that is one of the first identified cell-permeable, P-site inhibitors of adenylate cyclase. It inhibits forskolin-induced activation of a cAMP-dependent reporter gene in HEK293 cells with an IC50 value of 33 µM.{32386}
Brand:CaymanSKU:20358 -Available on backorder
2′,5′-dideoxy Adenosine is a nucleoside analog that is one of the first identified cell-permeable, P-site inhibitors of adenylate cyclase. It inhibits forskolin-induced activation of a cAMP-dependent reporter gene in HEK293 cells with an IC50 value of 33 µM.{32386}
Brand:CaymanSKU:20358 -Available on backorder
2′,5′-dideoxy Adenosine is a nucleoside analog that is one of the first identified cell-permeable, P-site inhibitors of adenylate cyclase. It inhibits forskolin-induced activation of a cAMP-dependent reporter gene in HEK293 cells with an IC50 value of 33 µM.{32386}
Brand:CaymanSKU:20358 -Available on backorder
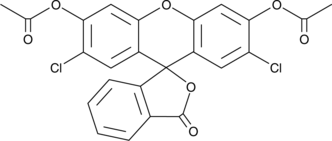
2′,7′-Dichlorofluorescein diacetate is as a cell-permeable fluorogenic probe to quantify reactive oxygen species (ROS) and nitric oxide (NO).{6536,40458} It is rapidly de-esterified in cells is oxidized to form fluorescent 2′,7′-dichlorofluorescein.{40459} 2’7-Dichlorofluorescein displays excitation/emission spectra of 492/515 nm.
Brand:CaymanSKU:20656 -Available on backorder
2′,7′-Dichlorofluorescein diacetate is as a cell-permeable fluorogenic probe to quantify reactive oxygen species (ROS) and nitric oxide (NO).{6536,40458} It is rapidly de-esterified in cells is oxidized to form fluorescent 2′,7′-dichlorofluorescein.{40459} 2’7-Dichlorofluorescein displays excitation/emission spectra of 492/515 nm.
Brand:CaymanSKU:20656 -Available on backorder
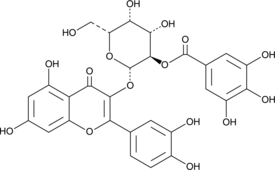
2”-O-Galloylhyperin is a natural flavonoid that has been found in P. incarnata and has diverse biological activities, including antioxidant, anti-inflammatory, hepatoprotective, and transporter-inhibiting properties.{53103,53104,53105} It scavenges 2,2-diphenyl-1-picrylhydrazyl (DPPH; Item No. 14805) and ABTS (Item No. 27317) radicals (IC50s = 3 and 3.5 μM, respectively).{53103} It also inhibits human organic anion transporting polypeptide 1B1 (OATP1B1; IC50 = 19 μM in HEK293 cells).{53104} 2”-O-Galloylhyperin (15 μM) decreases LPS-induced IL-6 and TNF-α cytokine production from mouse RAW 264.7 macrophages.{53105} It also decreases LPS-induced mortality and liver damage in a mouse model of septic shock when administered at a dose of 50 mg/kg.
Brand:CaymanSKU:29086 - 1 mgAvailable on backorder
2”-O-Galloylhyperin is a natural flavonoid that has been found in P. incarnata and has diverse biological activities, including antioxidant, anti-inflammatory, hepatoprotective, and transporter-inhibiting properties.{53103,53104,53105} It scavenges 2,2-diphenyl-1-picrylhydrazyl (DPPH; Item No. 14805) and ABTS (Item No. 27317) radicals (IC50s = 3 and 3.5 μM, respectively).{53103} It also inhibits human organic anion transporting polypeptide 1B1 (OATP1B1; IC50 = 19 μM in HEK293 cells).{53104} 2”-O-Galloylhyperin (15 μM) decreases LPS-induced IL-6 and TNF-α cytokine production from mouse RAW 264.7 macrophages.{53105} It also decreases LPS-induced mortality and liver damage in a mouse model of septic shock when administered at a dose of 50 mg/kg.
Brand:CaymanSKU:29086 - 10 mgAvailable on backorder
2”-O-Galloylhyperin is a natural flavonoid that has been found in P. incarnata and has diverse biological activities, including antioxidant, anti-inflammatory, hepatoprotective, and transporter-inhibiting properties.{53103,53104,53105} It scavenges 2,2-diphenyl-1-picrylhydrazyl (DPPH; Item No. 14805) and ABTS (Item No. 27317) radicals (IC50s = 3 and 3.5 μM, respectively).{53103} It also inhibits human organic anion transporting polypeptide 1B1 (OATP1B1; IC50 = 19 μM in HEK293 cells).{53104} 2”-O-Galloylhyperin (15 μM) decreases LPS-induced IL-6 and TNF-α cytokine production from mouse RAW 264.7 macrophages.{53105} It also decreases LPS-induced mortality and liver damage in a mouse model of septic shock when administered at a dose of 50 mg/kg.
Brand:CaymanSKU:29086 - 25 mgAvailable on backorder
2”-O-Galloylhyperin is a natural flavonoid that has been found in P. incarnata and has diverse biological activities, including antioxidant, anti-inflammatory, hepatoprotective, and transporter-inhibiting properties.{53103,53104,53105} It scavenges 2,2-diphenyl-1-picrylhydrazyl (DPPH; Item No. 14805) and ABTS (Item No. 27317) radicals (IC50s = 3 and 3.5 μM, respectively).{53103} It also inhibits human organic anion transporting polypeptide 1B1 (OATP1B1; IC50 = 19 μM in HEK293 cells).{53104} 2”-O-Galloylhyperin (15 μM) decreases LPS-induced IL-6 and TNF-α cytokine production from mouse RAW 264.7 macrophages.{53105} It also decreases LPS-induced mortality and liver damage in a mouse model of septic shock when administered at a dose of 50 mg/kg.
Brand:CaymanSKU:29086 - 5 mgAvailable on backorder
Cyclic GMP-AMP synthase (cGAS) is a cytosolic DNA sensor that detects the presence of nucleic acids in the cytosol of mammalian cells as an indicator of bacterial or viral infection.{22400} cGAS catalyzes the synthesis of a second messenger, 2’3’-cGAMP, from cytosolic ATP and GTP in response to dsDNA binding. 2’3’-cGAMP then binds tightly to the adaptor protein STING (stimulator of interferon genes), resulting in the recruitment of TBK1 and subsequent IRF3 phosphorylation.{35370} IRF3 induces the transcription and translation type I interferon, a potent antiviral cytokine.{35369} Activation of cGAS and the production of 2’3’-cGAMP are important in host defense, but also may play role in autoimmune or inflammatory diseases. Modulation of cGAS activity, with subsequent inhibition of induction of 2’3’-cGAMP formation is an active target of pharmacological intervention.{35371} Powered by BIOLOG Life Science Institute.
Brand:CaymanSKU:501700 - 96 solid wellsAvailable on backorder
Cyclic GMP-AMP synthase (cGAS) is a cytosolic DNA sensor that detects the presence of nucleic acids in the cytosol of mammalian cells as an indicator of bacterial or viral infection.{22400} cGAS catalyzes the synthesis of a second messenger, 2’3’-cGAMP, from cytosolic ATP and GTP in response to dsDNA binding. 2’3’-cGAMP then binds tightly to the adaptor protein STING (stimulator of interferon genes), resulting in the recruitment of TBK1 and subsequent IRF3 phosphorylation.{35370} IRF3 induces the transcription and translation type I interferon, a potent antiviral cytokine.{35369} Activation of cGAS and the production of 2’3’-cGAMP are important in host defense, but also may play role in autoimmune or inflammatory diseases. Modulation of cGAS activity, with subsequent inhibition of induction of 2’3’-cGAMP formation is an active target of pharmacological intervention.{35371} Powered by BIOLOG Life Science Institute.
Brand:CaymanSKU:501700 - 96 strip wellsAvailable on backorder
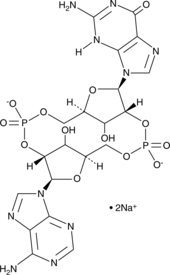
2’2’-cGAMP is a synthetic dinucleotide (CDN) that contains non-canonical 2’5′-phosphodiester bonds.{29220} It binds to the adapter protein stimulator of interferon genes (STING; Item Nos. 22816 | 22815), which is a component of the innate immune response that activates the type I interferon pathway when bound to cyclic dinucleotides. 2’2-cGAMP shows weaker binding to STING than 2’3’-cGAMP (Item No. 19887; Kd = 287 and 3.79 nM, respectively) but binds more strongly than 3’3’-cGAMP (Item No. 17966), cyclic di-GMP (Item No. 17144), or 3’2’-cGAMP, which bind in the micromolar range (Kds = 1.04, 1.21, or 1.61 µM, respectively).{29220} Despite weaker binding, 2’2’-cGAMP induces IFN-β production in the same concentration range as 2’3’-cGAMP (EC50s = 15.8 and 19.4 nM, respectively, in L929 cells).
Brand:CaymanSKU:22419 -Out of stock
2’2’-cGAMP is a synthetic dinucleotide (CDN) that contains non-canonical 2’5′-phosphodiester bonds.{29220} It binds to the adapter protein stimulator of interferon genes (STING; Item Nos. 22816 | 22815), which is a component of the innate immune response that activates the type I interferon pathway when bound to cyclic dinucleotides. 2’2-cGAMP shows weaker binding to STING than 2’3’-cGAMP (Item No. 19887; Kd = 287 and 3.79 nM, respectively) but binds more strongly than 3’3’-cGAMP (Item No. 17966), cyclic di-GMP (Item No. 17144), or 3’2’-cGAMP, which bind in the micromolar range (Kds = 1.04, 1.21, or 1.61 µM, respectively).{29220} Despite weaker binding, 2’2’-cGAMP induces IFN-β production in the same concentration range as 2’3’-cGAMP (EC50s = 15.8 and 19.4 nM, respectively, in L929 cells).
Brand:CaymanSKU:22419 -Out of stock
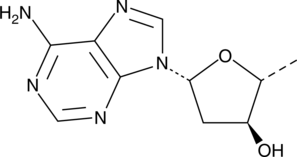
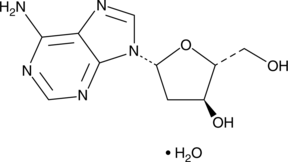
2′-Deoxyadenosine is a deoxyribonucleoside and an intermediate in the purine nucleotide degradation pathway.{51076} It is transported into cells via facilitated diffusion or formed within cells by degradation of S-adenosylhomocysteine or AMP and is removed from cells by purine metabolism or is converted into adenine nucleotides. 2′-Deoxyadenosine has been used in the characterization of DNA conformations and the synthesis of nucleoside analogs as antiviral agents.{51077,51078}
Brand:CaymanSKU:27315 - 10 gAvailable on backorder
2′-Deoxyadenosine is a deoxyribonucleoside and an intermediate in the purine nucleotide degradation pathway.{51076} It is transported into cells via facilitated diffusion or formed within cells by degradation of S-adenosylhomocysteine or AMP and is removed from cells by purine metabolism or is converted into adenine nucleotides. 2′-Deoxyadenosine has been used in the characterization of DNA conformations and the synthesis of nucleoside analogs as antiviral agents.{51077,51078}
Brand:CaymanSKU:27315 - 25 gAvailable on backorder
2′-Deoxyadenosine is a deoxyribonucleoside and an intermediate in the purine nucleotide degradation pathway.{51076} It is transported into cells via facilitated diffusion or formed within cells by degradation of S-adenosylhomocysteine or AMP and is removed from cells by purine metabolism or is converted into adenine nucleotides. 2′-Deoxyadenosine has been used in the characterization of DNA conformations and the synthesis of nucleoside analogs as antiviral agents.{51077,51078}
Brand:CaymanSKU:27315 - 5 gAvailable on backorder
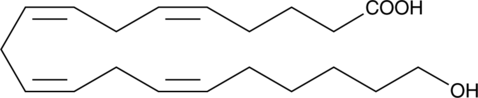
20-carboxy Arachidonic acid (20-COOH-AA) is the major metabolite of 20-HETE that is produced in renal tubular epithelial, endothelial, and microvascular smooth muscle cell cultures. This ω-oxidation conversion can take place using purified alcohol dehydrogenases three and four or by microsomes containing recombinant human CYP4F3B.{14780} Like 20-HETE, 20-COOH-AA inhibits ion transport in the kidneys. It also produces vasorelaxation of porcine coronary microvessels constricted with endothelin. 20-COOH-AA binds to isolated ligand binding domains of peroxisome proliferator-activated receptor α (PPARα) (Kd = 0.87 ± 0.12 µM) and PPARγ (Kd = 1.7 ± 0.5 µM), and is a dual activator of PPARα and PPARγ in a transiently transfected COS-7 cell reporter system.{14780}
Brand:CaymanSKU:10007912 - 100 µgAvailable on backorder
20-carboxy Arachidonic acid (20-COOH-AA) is the major metabolite of 20-HETE that is produced in renal tubular epithelial, endothelial, and microvascular smooth muscle cell cultures. This ω-oxidation conversion can take place using purified alcohol dehydrogenases three and four or by microsomes containing recombinant human CYP4F3B.{14780} Like 20-HETE, 20-COOH-AA inhibits ion transport in the kidneys. It also produces vasorelaxation of porcine coronary microvessels constricted with endothelin. 20-COOH-AA binds to isolated ligand binding domains of peroxisome proliferator-activated receptor α (PPARα) (Kd = 0.87 ± 0.12 µM) and PPARγ (Kd = 1.7 ± 0.5 µM), and is a dual activator of PPARα and PPARγ in a transiently transfected COS-7 cell reporter system.{14780}
Brand:CaymanSKU:10007912 - 25 µgAvailable on backorder
20-carboxy Arachidonic acid (20-COOH-AA) is the major metabolite of 20-HETE that is produced in renal tubular epithelial, endothelial, and microvascular smooth muscle cell cultures. This ω-oxidation conversion can take place using purified alcohol dehydrogenases three and four or by microsomes containing recombinant human CYP4F3B.{14780} Like 20-HETE, 20-COOH-AA inhibits ion transport in the kidneys. It also produces vasorelaxation of porcine coronary microvessels constricted with endothelin. 20-COOH-AA binds to isolated ligand binding domains of peroxisome proliferator-activated receptor α (PPARα) (Kd = 0.87 ± 0.12 µM) and PPARγ (Kd = 1.7 ± 0.5 µM), and is a dual activator of PPARα and PPARγ in a transiently transfected COS-7 cell reporter system.{14780}
Brand:CaymanSKU:10007912 - 50 µgAvailable on backorder
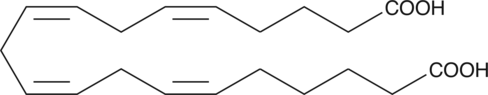
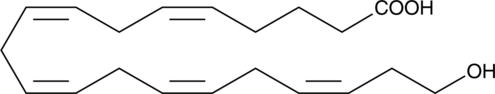
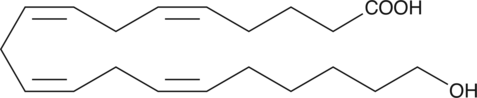
20-carboxy LTB4 is a metabolite of LTB4 in human neutrophils. In human leukocytes, LTB4 is inactivated by the enzyme LTB4 20-hydroxylase. The resulting 20-hydroxy LTB4 is further oxidized to 20-carboxy LTB4.{377} LTB4 metabolism in isolated rat hepatocytes also results in production of 20-carboxy LTB4 along with other ω-oxidation products.{1032} The biological activity of 20-carboxy LTB4 is only about 2.6% compared to that of LTB4 in causing PMNL degranulation.{399}
Brand:CaymanSKU:20180 -Available on backorder
20-carboxy LTB4 is a metabolite of LTB4 in human neutrophils. In human leukocytes, LTB4 is inactivated by the enzyme LTB4 20-hydroxylase. The resulting 20-hydroxy LTB4 is further oxidized to 20-carboxy LTB4.{377} LTB4 metabolism in isolated rat hepatocytes also results in production of 20-carboxy LTB4 along with other ω-oxidation products.{1032} The biological activity of 20-carboxy LTB4 is only about 2.6% compared to that of LTB4 in causing PMNL degranulation.{399}
Brand:CaymanSKU:20180 -Available on backorder
20-carboxy LTB4 is a metabolite of LTB4 in human neutrophils. In human leukocytes, LTB4 is inactivated by the enzyme LTB4 20-hydroxylase. The resulting 20-hydroxy LTB4 is further oxidized to 20-carboxy LTB4.{377} LTB4 metabolism in isolated rat hepatocytes also results in production of 20-carboxy LTB4 along with other ω-oxidation products.{1032} The biological activity of 20-carboxy LTB4 is only about 2.6% compared to that of LTB4 in causing PMNL degranulation.{399}
Brand:CaymanSKU:20180 -Available on backorder
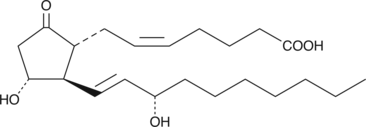
20-ethyl Prostaglandin E2 (20-ethyl PGE2) is an analog of PGE2 in which the ω-chain has been extended by the addition of two methylene carbon atoms. The only well studied prostaglandin analog with this structural feature is unoprostone, an F-series prostaglandin that is clinically approved as a glaucoma medication.{8147} Unoprostone also contains lower side chain modifications (13,14-dihydro-15-keto) which severely limit its affinity for FP receptors, contributing to its lack of potency as a medication. 20-ethyl PGE2 retains the natural 15(S) allylic hydroxyl in the lower side chain, which may improve its potency relative to unoprostone. However, ligand binding assays of this analog with respect to EP or other prostanoid receptors have not been published. E-type prostaglandins have been widely reported to have inflammatory,{5381} cytoprotective,{2216} and a variety of other effects.{1558}
Brand:CaymanSKU:-20-ethyl Prostaglandin E2 (20-ethyl PGE2) is an analog of PGE2 in which the ω-chain has been extended by the addition of two methylene carbon atoms. The only well studied prostaglandin analog with this structural feature is unoprostone, an F-series prostaglandin that is clinically approved as a glaucoma medication.{8147} Unoprostone also contains lower side chain modifications (13,14-dihydro-15-keto) which severely limit its affinity for FP receptors, contributing to its lack of potency as a medication. 20-ethyl PGE2 retains the natural 15(S) allylic hydroxyl in the lower side chain, which may improve its potency relative to unoprostone. However, ligand binding assays of this analog with respect to EP or other prostanoid receptors have not been published. E-type prostaglandins have been widely reported to have inflammatory,{5381} cytoprotective,{2216} and a variety of other effects.{1558}
Brand:CaymanSKU:-20-ethyl Prostaglandin E2 (20-ethyl PGE2) is an analog of PGE2 in which the ω-chain has been extended by the addition of two methylene carbon atoms. The only well studied prostaglandin analog with this structural feature is unoprostone, an F-series prostaglandin that is clinically approved as a glaucoma medication.{8147} Unoprostone also contains lower side chain modifications (13,14-dihydro-15-keto) which severely limit its affinity for FP receptors, contributing to its lack of potency as a medication. 20-ethyl PGE2 retains the natural 15(S) allylic hydroxyl in the lower side chain, which may improve its potency relative to unoprostone. However, ligand binding assays of this analog with respect to EP or other prostanoid receptors have not been published. E-type prostaglandins have been widely reported to have inflammatory,{5381} cytoprotective,{2216} and a variety of other effects.{1558}
Brand:CaymanSKU:-20-ethyl Prostaglandin F2α (20-ethyl PGF2α) is an analog of PGF2α in which the ω-chain has been extended by the addition of two more methylene carbon atoms. It is therefore a modified version of the clinically approved glaucoma medication unoprostone.{8147} Unoprostone also contains lower side chain modifications (13,14-dihydro-15-keto) which severely limit its affinity for FP receptors, contributing to its lack of potency as a medication. 20-ethyl PGF2α retains the natural 15(S) allylic hydroxyl in the lower side chain, which may improve its potency as an intraocular hypotensive agent compared to unoprostone. The 2 carbon extension in 20-ethyl-PGF2α increases the Ki (120 nM) for the FP receptor from bovine corpus luteum only about 2.5-fold compared to PGF2α (50 nM).{11182} In vivo effects may be prolonged using 20-ethyl PGF2α, as the activity of 15-hydroxy PGDH using 20-ethyl PGF2α as a substrate is only 35% of the activity observed with PGF2α.{2309,11182}
Brand:CaymanSKU:-Out of stock
20-ethyl Prostaglandin F2α (20-ethyl PGF2α) is an analog of PGF2α in which the ω-chain has been extended by the addition of two more methylene carbon atoms. It is therefore a modified version of the clinically approved glaucoma medication unoprostone.{8147} Unoprostone also contains lower side chain modifications (13,14-dihydro-15-keto) which severely limit its affinity for FP receptors, contributing to its lack of potency as a medication. 20-ethyl PGF2α retains the natural 15(S) allylic hydroxyl in the lower side chain, which may improve its potency as an intraocular hypotensive agent compared to unoprostone. The 2 carbon extension in 20-ethyl-PGF2α increases the Ki (120 nM) for the FP receptor from bovine corpus luteum only about 2.5-fold compared to PGF2α (50 nM).{11182} In vivo effects may be prolonged using 20-ethyl PGF2α, as the activity of 15-hydroxy PGDH using 20-ethyl PGF2α as a substrate is only 35% of the activity observed with PGF2α.{2309,11182}
Brand:CaymanSKU:-Out of stock
20-ethyl Prostaglandin F2α (20-ethyl PGF2α) is an analog of PGF2α in which the ω-chain has been extended by the addition of two more methylene carbon atoms. It is therefore a modified version of the clinically approved glaucoma medication unoprostone.{8147} Unoprostone also contains lower side chain modifications (13,14-dihydro-15-keto) which severely limit its affinity for FP receptors, contributing to its lack of potency as a medication. 20-ethyl PGF2α retains the natural 15(S) allylic hydroxyl in the lower side chain, which may improve its potency as an intraocular hypotensive agent compared to unoprostone. The 2 carbon extension in 20-ethyl-PGF2α increases the Ki (120 nM) for the FP receptor from bovine corpus luteum only about 2.5-fold compared to PGF2α (50 nM).{11182} In vivo effects may be prolonged using 20-ethyl PGF2α, as the activity of 15-hydroxy PGDH using 20-ethyl PGF2α as a substrate is only 35% of the activity observed with PGF2α.{2309,11182}
Brand:CaymanSKU:-Out of stock
20-HEPE is a metabolite of eicosapentaenoic acid (EPA; Item Nos. 90110 | 21908 | 90110.1) that is formed via ω-oxidation of EPA by cytochrome P450 (CYP) ω-oxidases, including human CYP4F3B.{37628} It activates peroxisome proliferator-activated receptor α (PPARα) in COS-7 cells expressing a luciferase reporter when used at a concentration of 10 μM. 20-HEPE also activates murine transient receptor potential vanilloid receptor 1 (mTRPV1) in vitro but lacks antinociceptive activity in rats.{37629}
Brand:CaymanSKU:-Available on backorder
20-HEPE is a metabolite of eicosapentaenoic acid (EPA; Item Nos. 90110 | 21908 | 90110.1) that is formed via ω-oxidation of EPA by cytochrome P450 (CYP) ω-oxidases, including human CYP4F3B.{37628} It activates peroxisome proliferator-activated receptor α (PPARα) in COS-7 cells expressing a luciferase reporter when used at a concentration of 10 μM. 20-HEPE also activates murine transient receptor potential vanilloid receptor 1 (mTRPV1) in vitro but lacks antinociceptive activity in rats.{37629}
Brand:CaymanSKU:-Available on backorder
20-HEPE is a metabolite of eicosapentaenoic acid (EPA; Item Nos. 90110 | 21908 | 90110.1) that is formed via ω-oxidation of EPA by cytochrome P450 (CYP) ω-oxidases, including human CYP4F3B.{37628} It activates peroxisome proliferator-activated receptor α (PPARα) in COS-7 cells expressing a luciferase reporter when used at a concentration of 10 μM. 20-HEPE also activates murine transient receptor potential vanilloid receptor 1 (mTRPV1) in vitro but lacks antinociceptive activity in rats.{37629}
Brand:CaymanSKU:-Available on backorder
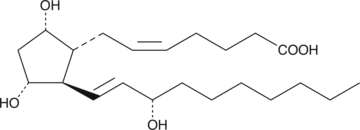
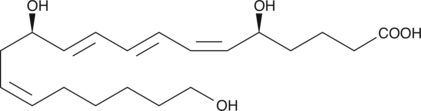
20-HETE is a cytochrome P450 (CYP450) metabolite postulated to play an autacoid role in the renal and cerebral vasculature.{7827} In rat cerebral microvessels, 20-HETE is a vasoconstrictor that mediates pressure-induced autoregulatory vasoconstriction.{9023} 20-HETE is excreted mainly as the glucuronide conjugate. The concentration of free 20-HETE (20-40 pg/ml in human urine) is about 10-fold lower than the corresponding concentration of the 20-glucuronide.{1119} 20-HETE can be further metabolized by cyclooxygenase to 20-hydroxy PGG2 and 20-hydroxy PGH2.{380}
Brand:CaymanSKU:90030 - 100 µgAvailable on backorder
20-HETE is a cytochrome P450 (CYP450) metabolite postulated to play an autacoid role in the renal and cerebral vasculature.{7827} In rat cerebral microvessels, 20-HETE is a vasoconstrictor that mediates pressure-induced autoregulatory vasoconstriction.{9023} 20-HETE is excreted mainly as the glucuronide conjugate. The concentration of free 20-HETE (20-40 pg/ml in human urine) is about 10-fold lower than the corresponding concentration of the 20-glucuronide.{1119} 20-HETE can be further metabolized by cyclooxygenase to 20-hydroxy PGG2 and 20-hydroxy PGH2.{380}
Brand:CaymanSKU:90030 - 25 µgAvailable on backorder
20-HETE is a cytochrome P450 (CYP450) metabolite postulated to play an autacoid role in the renal and cerebral vasculature.{7827} In rat cerebral microvessels, 20-HETE is a vasoconstrictor that mediates pressure-induced autoregulatory vasoconstriction.{9023} 20-HETE is excreted mainly as the glucuronide conjugate. The concentration of free 20-HETE (20-40 pg/ml in human urine) is about 10-fold lower than the corresponding concentration of the 20-glucuronide.{1119} 20-HETE can be further metabolized by cyclooxygenase to 20-hydroxy PGG2 and 20-hydroxy PGH2.{380}
Brand:CaymanSKU:90030 - 50 µgAvailable on backorder
20-HETE is a cytochrome P450 (CYP450) metabolite postulated to play an autacoid role in the renal and cerebral vasculature.{7827} In rat cerebral microvessels, 20-HETE is a vasoconstrictor that mediates pressure-induced autoregulatory vasoconstriction.{9023} 20-HETE is excreted mainly as the glucuronide conjugate. The concentration of free 20-HETE (20-40 pg/ml in human urine) is about 10-fold lower than the corresponding concentration of the 20-glucuronide.{1119} 20-HETE can be further metabolized by cyclooxygenase to 20-hydroxy PGG2 and 20-hydroxy PGH2.{380}
Brand:CaymanSKU:90030 - 500 µgAvailable on backorder
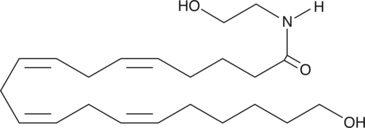
Arachidonoyl ethanolamide (AEA) is an endogenous lipid neurotransmitter with cannabinergic activity, binding both the central cannabinoid (CB1) and peripheral cannabinoid (CB2) receptors.{2713,14062} Fatty acid amide hydrolase (FAAH) is the enzyme responsible for the hydrolysis and inactivation of AEA.{13132} Metabolism of AEA by cyclooxygenase-2, leading to formation of prostaglandin ethanolamides, and by lipoxygenases has also been documented.{12168} 20-HETE ethanolamide is a potential cytochrome P450 metabolite of arachidonoyl ethanolamide, which may be particularly relevant under conditions of fatty acid amide hydrolase inhibition. Evidence for the formation of 20-HETE ethanolamide in vivo has not been documented.
Brand:CaymanSKU:10008602 - 100 µgAvailable on backorder
Arachidonoyl ethanolamide (AEA) is an endogenous lipid neurotransmitter with cannabinergic activity, binding both the central cannabinoid (CB1) and peripheral cannabinoid (CB2) receptors.{2713,14062} Fatty acid amide hydrolase (FAAH) is the enzyme responsible for the hydrolysis and inactivation of AEA.{13132} Metabolism of AEA by cyclooxygenase-2, leading to formation of prostaglandin ethanolamides, and by lipoxygenases has also been documented.{12168} 20-HETE ethanolamide is a potential cytochrome P450 metabolite of arachidonoyl ethanolamide, which may be particularly relevant under conditions of fatty acid amide hydrolase inhibition. Evidence for the formation of 20-HETE ethanolamide in vivo has not been documented.
Brand:CaymanSKU:10008602 - 25 µgAvailable on backorder
Arachidonoyl ethanolamide (AEA) is an endogenous lipid neurotransmitter with cannabinergic activity, binding both the central cannabinoid (CB1) and peripheral cannabinoid (CB2) receptors.{2713,14062} Fatty acid amide hydrolase (FAAH) is the enzyme responsible for the hydrolysis and inactivation of AEA.{13132} Metabolism of AEA by cyclooxygenase-2, leading to formation of prostaglandin ethanolamides, and by lipoxygenases has also been documented.{12168} 20-HETE ethanolamide is a potential cytochrome P450 metabolite of arachidonoyl ethanolamide, which may be particularly relevant under conditions of fatty acid amide hydrolase inhibition. Evidence for the formation of 20-HETE ethanolamide in vivo has not been documented.
Brand:CaymanSKU:10008602 - 50 µgAvailable on backorder
20-HETE is a cytochrome P450 (CYP450) metabolite postulated to play an autacoid role in the renal and cerebral vasculature.{7827} In rat cerebral microvessels, 20-HETE is a vasoconstrictor that mediates pressure-induced autoregulatory vasoconstriction.{9023} 20-HETE is excreted mainly as the glucuronide conjugate. The concentration of free 20-HETE (20-40 pg/ml in human urine) is about 10-fold lower than the corresponding concentration of the 20-glucuronide.{1119} 20-HETE can be further metabolized by cyclooxygenase to 20-hydroxy PGG2 and 20-hydroxy PGH2.{380} 20-HETE MaxSpec® standard is a quantitative grade standard of 20-HETE (Item No. 90030) that has been prepared specifically for mass spectrometry or any application where quantitative reproducibility is required. The solution has been prepared gravimetrically and is supplied in a deactivated glass ampule sealed under argon. The concentration was verified by comparison to an independently prepared calibration standard. This 20-HETE MaxSpec® standard is guaranteed to meet identity, purity, stability, and concentration specifications and is provided with a batch-specific certificate of analysis. Ongoing stability testing is performed to ensure the concentration remains accurate throughout the shelf life of the product. Note: The amount of solution added to the vial is in excess of the listed amount. Therefore, it is necessary to accurately measure volumes for preparation of calibration standards. Follow recommended storage and handling conditions to maintain product quality.
Brand:CaymanSKU:10007269 - 10 µgAvailable on backorder
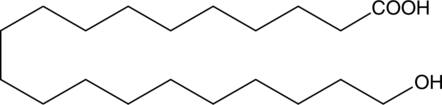
20-hydroxy Arachidic acid is a hydroxylated fatty acid that has been found in the suberin component of silver birch (B. pendula) outer bark as well as the leaves, roots, and wood of Q. ilex from Cala Violina and Colognole, Italy.{38894,38895} 20-hydroxy Arachidic acid was identified as a potential inhibitor of human rhinovirus coat protein in a structure-based virtual screen.{38896} [Matreya, LLC. Catalog No. 1877]
Brand:CaymanSKU:24652 - 10 mgAvailable on backorder
20-hydroxy Arachidic acid is a hydroxylated fatty acid that has been found in the suberin component of silver birch (B. pendula) outer bark as well as the leaves, roots, and wood of Q. ilex from Cala Violina and Colognole, Italy.{38894,38895} 20-hydroxy Arachidic acid was identified as a potential inhibitor of human rhinovirus coat protein in a structure-based virtual screen.{38896} [Matreya, LLC. Catalog No. 1877]
Brand:CaymanSKU:24652 - 25 mgAvailable on backorder
20-hydroxy Arachidic acid is a hydroxylated fatty acid that has been found in the suberin component of silver birch (B. pendula) outer bark as well as the leaves, roots, and wood of Q. ilex from Cala Violina and Colognole, Italy.{38894,38895} 20-hydroxy Arachidic acid was identified as a potential inhibitor of human rhinovirus coat protein in a structure-based virtual screen.{38896} [Matreya, LLC. Catalog No. 1877]
Brand:CaymanSKU:24652 - 5 mgAvailable on backorder
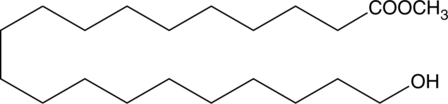
20-hydroxy Arachidic acid methyl ester is a hydroxylated fatty acid methyl ester that has been found in P. reticulata cork extracts as well as peat samples from inland Florida.{39675,38856} [Matreya, LLC. Catalog No. 1878]
Brand:CaymanSKU:24653 - 10 mgAvailable on backorder
20-hydroxy Arachidic acid methyl ester is a hydroxylated fatty acid methyl ester that has been found in P. reticulata cork extracts as well as peat samples from inland Florida.{39675,38856} [Matreya, LLC. Catalog No. 1878]
Brand:CaymanSKU:24653 - 25 mgAvailable on backorder
20-hydroxy Arachidic acid methyl ester is a hydroxylated fatty acid methyl ester that has been found in P. reticulata cork extracts as well as peat samples from inland Florida.{39675,38856} [Matreya, LLC. Catalog No. 1878]
Brand:CaymanSKU:24653 - 5 mgAvailable on backorder
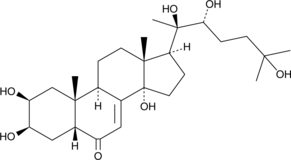
20-hydroxy Ecdysone is an ecdysteroid hormone produced in arthropod species that induces developmental changes associated with ecdysis and the completion of metamorphosis.{26314} It is also produced in plants as a defensive strategy to disrupt the development of insect pests.{26312} In target tissues, 20-hydroxy ecdysone binds to the nuclear hormone receptor, ecdysone receptor, initiating a signaling cascade that results in cell cycle arrest.{26314,26313} Ecdysteroids such as this compound have been employed as tools in inducible gene regulation systems controlled by reporter cells transfected with the ecdysone receptor.{26316,26315}
Brand:CaymanSKU:-20-hydroxy Ecdysone is an ecdysteroid hormone produced in arthropod species that induces developmental changes associated with ecdysis and the completion of metamorphosis.{26314} It is also produced in plants as a defensive strategy to disrupt the development of insect pests.{26312} In target tissues, 20-hydroxy ecdysone binds to the nuclear hormone receptor, ecdysone receptor, initiating a signaling cascade that results in cell cycle arrest.{26314,26313} Ecdysteroids such as this compound have been employed as tools in inducible gene regulation systems controlled by reporter cells transfected with the ecdysone receptor.{26316,26315}
Brand:CaymanSKU:-20-hydroxy Ecdysone is an ecdysteroid hormone produced in arthropod species that induces developmental changes associated with ecdysis and the completion of metamorphosis.{26314} It is also produced in plants as a defensive strategy to disrupt the development of insect pests.{26312} In target tissues, 20-hydroxy ecdysone binds to the nuclear hormone receptor, ecdysone receptor, initiating a signaling cascade that results in cell cycle arrest.{26314,26313} Ecdysteroids such as this compound have been employed as tools in inducible gene regulation systems controlled by reporter cells transfected with the ecdysone receptor.{26316,26315}
Brand:CaymanSKU:-20-hydroxy Ecdysone is an ecdysteroid hormone produced in arthropod species that induces developmental changes associated with ecdysis and the completion of metamorphosis.{26314} It is also produced in plants as a defensive strategy to disrupt the development of insect pests.{26312} In target tissues, 20-hydroxy ecdysone binds to the nuclear hormone receptor, ecdysone receptor, initiating a signaling cascade that results in cell cycle arrest.{26314,26313} Ecdysteroids such as this compound have been employed as tools in inducible gene regulation systems controlled by reporter cells transfected with the ecdysone receptor.{26316,26315}
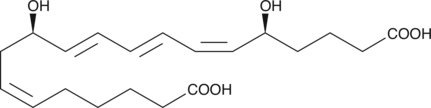
Brand:CaymanSKU:-20-hydroxy LTB4 is a metabolite of LTB4 in human neutrophils. In human leukocytes, LTB4 is inactivated by the enzyme LTB4 20-hydroxylase. 20-hydroxy LTB4 is not only much less active (~5%) compared to LTB4 in causing degranulation of PMNL,{399} but actually inhibits LTB4-induced degranulation of human neutrophils (Ki = 13.3 nM).{1106} However, 20-hydroxy LTB4 is as active as LTB4 in contracting parenchymal strips from guinea pig lung.{377} 20-hydroxy LTB4 retains considerable ligand binding affinity at the BLT2 receptor,{8743,9270} but does not appear to function as an agonist.{8525}
Brand:CaymanSKU:20190 -Available on backorder
20-hydroxy LTB4 is a metabolite of LTB4 in human neutrophils. In human leukocytes, LTB4 is inactivated by the enzyme LTB4 20-hydroxylase. 20-hydroxy LTB4 is not only much less active (~5%) compared to LTB4 in causing degranulation of PMNL,{399} but actually inhibits LTB4-induced degranulation of human neutrophils (Ki = 13.3 nM).{1106} However, 20-hydroxy LTB4 is as active as LTB4 in contracting parenchymal strips from guinea pig lung.{377} 20-hydroxy LTB4 retains considerable ligand binding affinity at the BLT2 receptor,{8743,9270} but does not appear to function as an agonist.{8525}
Brand:CaymanSKU:20190 -Available on backorder
20-hydroxy LTB4 is a metabolite of LTB4 in human neutrophils. In human leukocytes, LTB4 is inactivated by the enzyme LTB4 20-hydroxylase. 20-hydroxy LTB4 is not only much less active (~5%) compared to LTB4 in causing degranulation of PMNL,{399} but actually inhibits LTB4-induced degranulation of human neutrophils (Ki = 13.3 nM).{1106} However, 20-hydroxy LTB4 is as active as LTB4 in contracting parenchymal strips from guinea pig lung.{377} 20-hydroxy LTB4 retains considerable ligand binding affinity at the BLT2 receptor,{8743,9270} but does not appear to function as an agonist.{8525}
Brand:CaymanSKU:20190 -Available on backorder
20-hydroxy Prostaglandin E2 (20-hydroxy PGE2) is a product of cytochrome P450 metabolism of PGE2 (Item No. 14010).{4309,726} ω-Oxidation at C-20 followed by β-oxidation and the loss of up to four carbons from the lower side chain is a prominent metabolic pathway for PGE2. 20-hydroxy PGE2 is the putative first intermediate in this chain of chemical transformations.
Brand:CaymanSKU:-20-hydroxy Prostaglandin E2 (20-hydroxy PGE2) is a product of cytochrome P450 metabolism of PGE2 (Item No. 14010).{4309,726} ω-Oxidation at C-20 followed by β-oxidation and the loss of up to four carbons from the lower side chain is a prominent metabolic pathway for PGE2. 20-hydroxy PGE2 is the putative first intermediate in this chain of chemical transformations.
Brand:CaymanSKU:-20-hydroxy Prostaglandin E2 (20-hydroxy PGE2) is a product of cytochrome P450 metabolism of PGE2 (Item No. 14010).{4309,726} ω-Oxidation at C-20 followed by β-oxidation and the loss of up to four carbons from the lower side chain is a prominent metabolic pathway for PGE2. 20-hydroxy PGE2 is the putative first intermediate in this chain of chemical transformations.
Brand:CaymanSKU:-20-hydroxy Prostaglandin E2 (20-hydroxy PGE2) is a product of cytochrome P450 metabolism of PGE2 (Item No. 14010).{4309,726} ω-Oxidation at C-20 followed by β-oxidation and the loss of up to four carbons from the lower side chain is a prominent metabolic pathway for PGE2. 20-hydroxy PGE2 is the putative first intermediate in this chain of chemical transformations.
Brand:CaymanSKU:-20-hydroxy Prostaglandin F2α (20-hydroxy PGF2α) is the ω-oxidation product of PGF2α. Cultured type II alveolar cells from pregnant rabbits metabolize exogenous PGF2α via microsomal cytochrome P450 ω-oxidation, producing 20-hydroxy PGF2α and its 15-hydroxy PGDH metabolites. Cells from male rabbits exhibit only the 15-hydroxy PGDH pathway.{4308}
Brand:CaymanSKU:-Out of stock
20-hydroxy Prostaglandin F2α (20-hydroxy PGF2α) is the ω-oxidation product of PGF2α. Cultured type II alveolar cells from pregnant rabbits metabolize exogenous PGF2α via microsomal cytochrome P450 ω-oxidation, producing 20-hydroxy PGF2α and its 15-hydroxy PGDH metabolites. Cells from male rabbits exhibit only the 15-hydroxy PGDH pathway.{4308}
Brand:CaymanSKU:-Out of stock