Cayman
Showing 3751–3900 of 45550 results
-
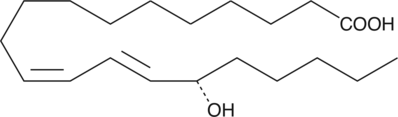
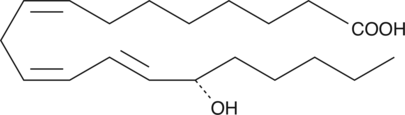
15(S)-HEDE is produced from 11Z,14Z-eicosadienoic acid by 15-LO. 15(S)-HEDE is an inhibitor of RBL-1 cell 5-LO with an IC50 value of 26 µM.{951}
Brand:CaymanSKU:37720 - 100 µgAvailable on backorder
-
15(S)-HEDE is produced from 11Z,14Z-eicosadienoic acid by 15-LO. 15(S)-HEDE is an inhibitor of RBL-1 cell 5-LO with an IC50 value of 26 µM.{951}
Brand:CaymanSKU:37720 - 25 µgAvailable on backorder
-
15(S)-HEDE is produced from 11Z,14Z-eicosadienoic acid by 15-LO. 15(S)-HEDE is an inhibitor of RBL-1 cell 5-LO with an IC50 value of 26 µM.{951}
Brand:CaymanSKU:37720 - 250 µgAvailable on backorder
-
15(S)-HEDE is produced from 11Z,14Z-eicosadienoic acid by 15-LO. 15(S)-HEDE is an inhibitor of RBL-1 cell 5-LO with an IC50 value of 26 µM.{951}
Brand:CaymanSKU:37720 - 50 µgAvailable on backorder
-
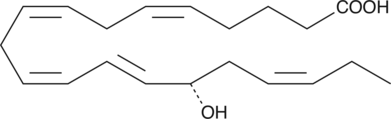
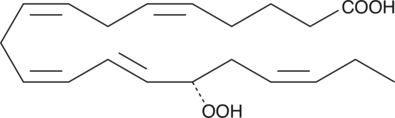
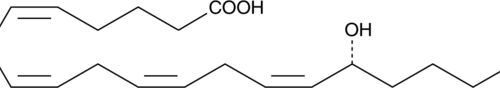
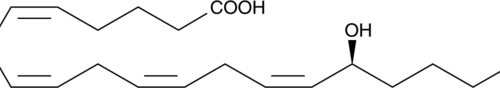
15(S)-HEPE is a monohydroxy fatty acid. 15(S)-HEPE is biosynthesized from eicosapentaenoic acid (Item Nos. 90110 | 90110.1 | 21908) by 15-lipoxygenase (15-LO).{6407,54203} Serum levels of 15(S)-HEPE are elevated in patients with asthma.{54204}
Brand:CaymanSKU:32710 - 100 µgAvailable on backorder
-
15(S)-HEPE is a monohydroxy fatty acid. 15(S)-HEPE is biosynthesized from eicosapentaenoic acid (Item Nos. 90110 | 90110.1 | 21908) by 15-lipoxygenase (15-LO).{6407,54203} Serum levels of 15(S)-HEPE are elevated in patients with asthma.{54204}
Brand:CaymanSKU:32710 - 25 µgAvailable on backorder
-
15(S)-HEPE is a monohydroxy fatty acid. 15(S)-HEPE is biosynthesized from eicosapentaenoic acid (Item Nos. 90110 | 90110.1 | 21908) by 15-lipoxygenase (15-LO).{6407,54203} Serum levels of 15(S)-HEPE are elevated in patients with asthma.{54204}
Brand:CaymanSKU:32710 - 250 µgAvailable on backorder
-
15(S)-HEPE is a monohydroxy fatty acid. 15(S)-HEPE is biosynthesized from eicosapentaenoic acid (Item Nos. 90110 | 90110.1 | 21908) by 15-lipoxygenase (15-LO).{6407,54203} Serum levels of 15(S)-HEPE are elevated in patients with asthma.{54204}
Brand:CaymanSKU:32710 - 50 µgAvailable on backorder
-
15(S)-HEPE is a monohydroxy fatty acid synthesized from EPA by the action of 15-LO. The biosynthesis of 15-HEPE from EPA in guinea pig epidermal enzyme preparations has been documented.{6407} 15(S)-HEPE generated by soybean lipoxygenation of EPA inhibits RBL-1 cell 5-LO with an IC50 value of 28 µM.{6407} 15(S)-HEPE MaxSpec® standard is a quantitative grade standard of 15(S)-HEPE (Item No. 32710) that has been prepared specifically for mass spectrometry and related applications where quantitative reproducibility is required. The solution has been prepared gravimetrically and is supplied in a deactivated glass ampule sealed under argon. The concentration was verified by comparison to an independently prepared calibration standard. This 15(S)-HEPE MaxSpec® standard is guaranteed to meet identity, purity, stability, and concentration specifications and is provided with a batch-specific certificate of analysis. Ongoing stability testing is performed to ensure the concentration remains accurate throughout the shelf life of the product. Note: The amount of solution added to the vial is in excess of the listed amount. Therefore, it is necessary to accurately measure volumes for preparation of calibration standards. Follow recommended storage and handling conditions to maintain product quality.
Brand:CaymanSKU:26003 - 10 µgAvailable on backorder
-
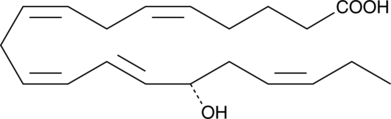
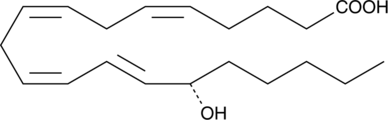
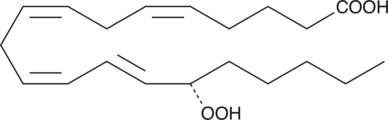
15(S)-HETE is a major arachidonic acid metabolite from the 15-lipoxygenase pathway. In mammals, 15(S)-HETE is synthesized in the respiratory epithelium, leukocytes, and reticulocytes.{118} 15(S)-HETE is present in µg/ml concentrations in the nasal secretions of allergic rhinitis.{373}
Brand:CaymanSKU:34720 - 100 µgAvailable on backorder
-
15(S)-HETE is a major arachidonic acid metabolite from the 15-lipoxygenase pathway. In mammals, 15(S)-HETE is synthesized in the respiratory epithelium, leukocytes, and reticulocytes.{118} 15(S)-HETE is present in µg/ml concentrations in the nasal secretions of allergic rhinitis.{373}
Brand:CaymanSKU:34720 - 25 µgAvailable on backorder
-
15(S)-HETE is a major arachidonic acid metabolite from the 15-lipoxygenase pathway. In mammals, 15(S)-HETE is synthesized in the respiratory epithelium, leukocytes, and reticulocytes.{118} 15(S)-HETE is present in µg/ml concentrations in the nasal secretions of allergic rhinitis.{373}
Brand:CaymanSKU:34720 - 50 µgAvailable on backorder
-
15(S)-HETE is a major arachidonic acid metabolite from the 15-lipoxygenase pathway. In mammals, 15(S)-HETE is synthesized in the respiratory epithelium, leukocytes, and reticulocytes.{118} 15(S)-HETE is present in µg/ml concentrations in the nasal secretions of allergic rhinitis.{373}
Brand:CaymanSKU:34720 - 500 µgAvailable on backorder
-
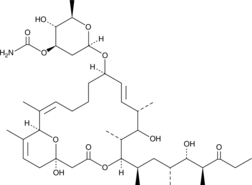
Brand:CaymanSKU:434722 - 100 dtn
Available on backorder
-
Brand:CaymanSKU:434722 - 500 dtn
Available on backorder
-
15(S)-HETE is produced from arachidonic acid by the enzyme 15-lipoxygenase (15-LO). In humans it is formed primarily in the respiratory epithelium, leukocytes, and reticulocytes.{2363} 15(S)-HETE has been detected in high concentrations in nasal secretions and may contribute to allergic rhinitis.{373} 15(S)-HETE has anti-inflammatory properties, inhibiting carrageenan-induced arthritis and lowering leukotriene B4 (LTB4) concentrations in the synovial fluid of dogs.{1014} It may regulate T-lymphocytes by inhibiting 5- and 12-LOs.{682} It is also a vasoconstrictor, constricting cerebral and coronary arteries of dogs in vitro and cerebral arteries of pigs in vivo.{1015} 15(S)-HETE may also play a role in cancer, inhibiting apoptosis by carcinosarcoma cells.{3814} Our 15(S)-HETE ELISA is a competitive assay that permits the measurement of 15(S)-HETE within the range of 78-10,000 pg/ml, typically with a sensitivity (80% B/B0) of 185.4 pg/ml. This assay is very specific for 15(S)-HETE, showing very low crossreactivity to other HETEs.
Brand:CaymanSKU:534721 - 480 solid wellsAvailable on backorder
-
15(S)-HETE is produced from arachidonic acid by the enzyme 15-lipoxygenase (15-LO). In humans it is formed primarily in the respiratory epithelium, leukocytes, and reticulocytes.{2363} 15(S)-HETE has been detected in high concentrations in nasal secretions and may contribute to allergic rhinitis.{373} 15(S)-HETE has anti-inflammatory properties, inhibiting carrageenan-induced arthritis and lowering leukotriene B4 (LTB4) concentrations in the synovial fluid of dogs.{1014} It may regulate T-lymphocytes by inhibiting 5- and 12-LOs.{682} It is also a vasoconstrictor, constricting cerebral and coronary arteries of dogs in vitro and cerebral arteries of pigs in vivo.{1015} 15(S)-HETE may also play a role in cancer, inhibiting apoptosis by carcinosarcoma cells.{3814} Our 15(S)-HETE ELISA is a competitive assay that permits the measurement of 15(S)-HETE within the range of 78-10,000 pg/ml, typically with a sensitivity (80% B/B0) of 185.4 pg/ml. This assay is very specific for 15(S)-HETE, showing very low crossreactivity to other HETEs.
Brand:CaymanSKU:534721 - 480 strip wellsAvailable on backorder
-
15(S)-HETE is produced from arachidonic acid by the enzyme 15-lipoxygenase (15-LO). In humans it is formed primarily in the respiratory epithelium, leukocytes, and reticulocytes.{2363} 15(S)-HETE has been detected in high concentrations in nasal secretions and may contribute to allergic rhinitis.{373} 15(S)-HETE has anti-inflammatory properties, inhibiting carrageenan-induced arthritis and lowering leukotriene B4 (LTB4) concentrations in the synovial fluid of dogs.{1014} It may regulate T-lymphocytes by inhibiting 5- and 12-LOs.{682} It is also a vasoconstrictor, constricting cerebral and coronary arteries of dogs in vitro and cerebral arteries of pigs in vivo.{1015} 15(S)-HETE may also play a role in cancer, inhibiting apoptosis by carcinosarcoma cells.{3814} Our 15(S)-HETE ELISA is a competitive assay that permits the measurement of 15(S)-HETE within the range of 78-10,000 pg/ml, typically with a sensitivity (80% B/B0) of 185.4 pg/ml. This assay is very specific for 15(S)-HETE, showing very low crossreactivity to other HETEs.
Brand:CaymanSKU:534721 - 96 solid wellsAvailable on backorder
-
15(S)-HETE is produced from arachidonic acid by the enzyme 15-lipoxygenase (15-LO). In humans it is formed primarily in the respiratory epithelium, leukocytes, and reticulocytes.{2363} 15(S)-HETE has been detected in high concentrations in nasal secretions and may contribute to allergic rhinitis.{373} 15(S)-HETE has anti-inflammatory properties, inhibiting carrageenan-induced arthritis and lowering leukotriene B4 (LTB4) concentrations in the synovial fluid of dogs.{1014} It may regulate T-lymphocytes by inhibiting 5- and 12-LOs.{682} It is also a vasoconstrictor, constricting cerebral and coronary arteries of dogs in vitro and cerebral arteries of pigs in vivo.{1015} 15(S)-HETE may also play a role in cancer, inhibiting apoptosis by carcinosarcoma cells.{3814} Our 15(S)-HETE ELISA is a competitive assay that permits the measurement of 15(S)-HETE within the range of 78-10,000 pg/ml, typically with a sensitivity (80% B/B0) of 185.4 pg/ml. This assay is very specific for 15(S)-HETE, showing very low crossreactivity to other HETEs.
Brand:CaymanSKU:534721 - 96 strip wellsAvailable on backorder
-
Brand:CaymanSKU:434724 - 1 ea
Available on backorder
-
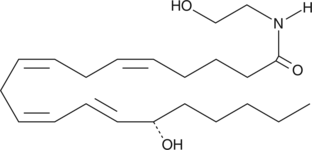
Arachidonoyl ethanolamide (AEA; Item No. 90050) was the first endogenous cannabinoid (CB) to be isolated and characterized as an agonist acting on the same receptors (CB1 and CB2) as THC.{1134,2713} Since that time, a number of related endocannabinoids have been isolated, most notably 2-arachidonoyl glycerol (Item No. 62160).{2713} Lipoxygenases, especially rabbit reticulocyte and soybean 15-lipoxygenases, actively convert endocannabinoids to their 15(S)-hydroperoxy and hydroxy metabolites.{11412} 15(S)-HETE ethanolamide is less potent than AEA at the CB1 receptor (Ki of 600 versus 90 nM). 15(S)-HETE ethanolamide also inhibits fatty acid amide hydrolase.{11365}
Brand:CaymanSKU:10169 - 100 µgAvailable on backorder
-
Arachidonoyl ethanolamide (AEA; Item No. 90050) was the first endogenous cannabinoid (CB) to be isolated and characterized as an agonist acting on the same receptors (CB1 and CB2) as THC.{1134,2713} Since that time, a number of related endocannabinoids have been isolated, most notably 2-arachidonoyl glycerol (Item No. 62160).{2713} Lipoxygenases, especially rabbit reticulocyte and soybean 15-lipoxygenases, actively convert endocannabinoids to their 15(S)-hydroperoxy and hydroxy metabolites.{11412} 15(S)-HETE ethanolamide is less potent than AEA at the CB1 receptor (Ki of 600 versus 90 nM). 15(S)-HETE ethanolamide also inhibits fatty acid amide hydrolase.{11365}
Brand:CaymanSKU:10169 - 25 µgAvailable on backorder
-
Arachidonoyl ethanolamide (AEA; Item No. 90050) was the first endogenous cannabinoid (CB) to be isolated and characterized as an agonist acting on the same receptors (CB1 and CB2) as THC.{1134,2713} Since that time, a number of related endocannabinoids have been isolated, most notably 2-arachidonoyl glycerol (Item No. 62160).{2713} Lipoxygenases, especially rabbit reticulocyte and soybean 15-lipoxygenases, actively convert endocannabinoids to their 15(S)-hydroperoxy and hydroxy metabolites.{11412} 15(S)-HETE ethanolamide is less potent than AEA at the CB1 receptor (Ki of 600 versus 90 nM). 15(S)-HETE ethanolamide also inhibits fatty acid amide hydrolase.{11365}
Brand:CaymanSKU:10169 - 50 µgAvailable on backorder
-
15(S)-HETE is a major arachidonic acid metabolite from the 15-lipoxygenase pathway. In mammals, 15(S)-HETE is synthesized in the respiratory epithelium, leukocytes, and reticulocytes.{118} 15(S)-HETE is present in µg/ml concentrations in the nasal secretions of allergic rhinitis.{373} 15(S)-HETE MaxSpec® standard is a quantitative grade standard of 15(S)-HETE (Item No. 34720) that has been prepared specifically for mass spectrometry and related applications where quantitative reproducibility is required. The solution has been prepared gravimetrically and is supplied in a deactivated glass ampule sealed under argon. The concentration was verified by comparison to an independently prepared calibration standard. This 15(S)-HETE MaxSpec® standard is guaranteed to meet identity, purity, stability, and concentration specifications and is provided with a batch-specific certificate of analysis. Ongoing stability testing is performed to ensure the concentration remains accurate throughout the shelf life of the product. Note: The amount of solution added to the vial is in excess of the listed amount. Therefore, it is necessary to accurately measure volumes for preparation of calibration standards. Follow recommended storage and handling conditions to maintain product quality.
Brand:CaymanSKU:10007251 - 100 µgAvailable on backorder
-
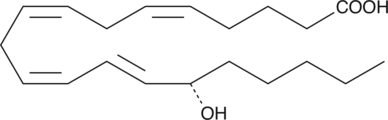
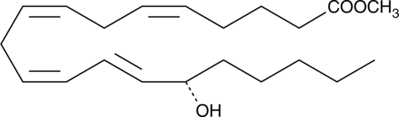
15(S)-HETE methyl ester is a synthetic derivative of 15(S)-HETE (Item No. 34720), a major arachidonic acid metabolite from the 15-lipoxygenase pathway. Methyl esters of lipids are commonly used in formulations of nutritional supplements.
Brand:CaymanSKU:21327 -Out of stock
-
15(S)-HETE methyl ester is a synthetic derivative of 15(S)-HETE (Item No. 34720), a major arachidonic acid metabolite from the 15-lipoxygenase pathway. Methyl esters of lipids are commonly used in formulations of nutritional supplements.
Brand:CaymanSKU:21327 -Out of stock
-
15(S)-HETE methyl ester is a synthetic derivative of 15(S)-HETE (Item No. 34720), a major arachidonic acid metabolite from the 15-lipoxygenase pathway. Methyl esters of lipids are commonly used in formulations of nutritional supplements.
Brand:CaymanSKU:21327 -Out of stock
-
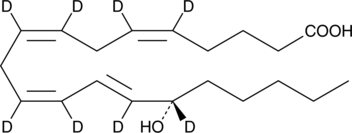
15(S)-HETE-d8 contains eight deuterium atoms at the 5, 6, 8, 9, 11, 12, 14, and 15 positions. It is intended for use as an internal standard for the quantification of 15(S)-HETE by GC- or LC-MS. 15(S)-HETE is a major arachidonic acid metabolite from the 15-lipoxygenase pathway. In mammals, 15(S)-HETE is synthesized in the respiratory epithelium, leukocytes, and reticulocytes.{118} 15(S)-HETE is present in µg/ml concentrations in the nasal secretions of allergic rhinitis.{373}
Brand:CaymanSKU:334720 - 100 µgAvailable on backorder
-
15(S)-HETE-d8 contains eight deuterium atoms at the 5, 6, 8, 9, 11, 12, 14, and 15 positions. It is intended for use as an internal standard for the quantification of 15(S)-HETE by GC- or LC-MS. 15(S)-HETE is a major arachidonic acid metabolite from the 15-lipoxygenase pathway. In mammals, 15(S)-HETE is synthesized in the respiratory epithelium, leukocytes, and reticulocytes.{118} 15(S)-HETE is present in µg/ml concentrations in the nasal secretions of allergic rhinitis.{373}
Brand:CaymanSKU:334720 - 25 µgAvailable on backorder
-
15(S)-HETE-d8 contains eight deuterium atoms at the 5, 6, 8, 9, 11, 12, 14, and 15 positions. It is intended for use as an internal standard for the quantification of 15(S)-HETE by GC- or LC-MS. 15(S)-HETE is a major arachidonic acid metabolite from the 15-lipoxygenase pathway. In mammals, 15(S)-HETE is synthesized in the respiratory epithelium, leukocytes, and reticulocytes.{118} 15(S)-HETE is present in µg/ml concentrations in the nasal secretions of allergic rhinitis.{373}
Brand:CaymanSKU:334720 - 50 µgAvailable on backorder
-
15(S)-HETrE is the hydroxy-trienoic acid resulting from 15-lipoxygenation of dihomo-γ-linolenic acid (DGLA; Item No. 90230). It is an inhibitor of 5-LO in human PMNL with an IC50 value of 4.6 µM.{2692} In RBL cells, 15(S)-HETrE inhibits 5-LO, but is about 1/20 as potent as 15(S)-HpETE.{951}
Brand:CaymanSKU:36720 - 100 µgAvailable on backorder
-
15(S)-HETrE is the hydroxy-trienoic acid resulting from 15-lipoxygenation of dihomo-γ-linolenic acid (DGLA; Item No. 90230). It is an inhibitor of 5-LO in human PMNL with an IC50 value of 4.6 µM.{2692} In RBL cells, 15(S)-HETrE inhibits 5-LO, but is about 1/20 as potent as 15(S)-HpETE.{951}
Brand:CaymanSKU:36720 - 25 µgAvailable on backorder
-
15(S)-HETrE is the hydroxy-trienoic acid resulting from 15-lipoxygenation of dihomo-γ-linolenic acid (DGLA; Item No. 90230). It is an inhibitor of 5-LO in human PMNL with an IC50 value of 4.6 µM.{2692} In RBL cells, 15(S)-HETrE inhibits 5-LO, but is about 1/20 as potent as 15(S)-HpETE.{951}
Brand:CaymanSKU:36720 - 50 µgAvailable on backorder
-
15(S)-HETrE is the hydroxy-trienoic acid resulting from 15-lipoxygenation of dihomo-γ-linolenic acid (DGLA; Item No. 90230). It is an inhibitor of 5-LO in human PMNL with an IC50 value of 4.6 µM.{2692} In RBL cells, 15(S)-HETrE inhibits 5-LO, but is about 1/20 as potent as 15(S)-HpETE.{951}
Brand:CaymanSKU:36720 - 500 µgAvailable on backorder
-
15(S)-HpEDE is a monohydroperoxy polyunsaturated fatty acid produced by the action of 15-lipoxygenase on eicosadienoic acid. Although the biological activities of 15(S)-HpEDE have not been well characterized, it is expected to behave similarly to 15(S)-HpETE (Cat. No. 44720).
Brand:CaymanSKU:47720 - 100 µgAvailable on backorder
-
15(S)-HpEDE is a monohydroperoxy polyunsaturated fatty acid produced by the action of 15-lipoxygenase on eicosadienoic acid. Although the biological activities of 15(S)-HpEDE have not been well characterized, it is expected to behave similarly to 15(S)-HpETE (Cat. No. 44720).
Brand:CaymanSKU:47720 - 25 µgAvailable on backorder
-
15(S)-HpEDE is a monohydroperoxy polyunsaturated fatty acid produced by the action of 15-lipoxygenase on eicosadienoic acid. Although the biological activities of 15(S)-HpEDE have not been well characterized, it is expected to behave similarly to 15(S)-HpETE (Cat. No. 44720).
Brand:CaymanSKU:47720 - 250 µgAvailable on backorder
-
15(S)-HpEDE is a monohydroperoxy polyunsaturated fatty acid produced by the action of 15-lipoxygenase on eicosadienoic acid. Although the biological activities of 15(S)-HpEDE have not been well characterized, it is expected to behave similarly to 15(S)-HpETE (Cat. No. 44720).
Brand:CaymanSKU:47720 - 50 µgAvailable on backorder
-
15(S)-HpEPE is a monohydroperoxy polyunsaturated fatty acid produced by the action of 15-lipoxygenase on eicosapentaenoic acid. Although the biological activities of 15(S)-HpEPE have not been well characterized, it is expected to behave similarly to 15(S)-HpETE (Catalog No. 44720).
Brand:CaymanSKU:42710 - 100 µgAvailable on backorder
-
15(S)-HpEPE is a monohydroperoxy polyunsaturated fatty acid produced by the action of 15-lipoxygenase on eicosapentaenoic acid. Although the biological activities of 15(S)-HpEPE have not been well characterized, it is expected to behave similarly to 15(S)-HpETE (Catalog No. 44720).
Brand:CaymanSKU:42710 - 25 µgAvailable on backorder
-
15(S)-HpEPE is a monohydroperoxy polyunsaturated fatty acid produced by the action of 15-lipoxygenase on eicosapentaenoic acid. Although the biological activities of 15(S)-HpEPE have not been well characterized, it is expected to behave similarly to 15(S)-HpETE (Catalog No. 44720).
Brand:CaymanSKU:42710 - 250 µgAvailable on backorder
-
15(S)-HpEPE is a monohydroperoxy polyunsaturated fatty acid produced by the action of 15-lipoxygenase on eicosapentaenoic acid. Although the biological activities of 15(S)-HpEPE have not been well characterized, it is expected to behave similarly to 15(S)-HpETE (Catalog No. 44720).
Brand:CaymanSKU:42710 - 50 µgAvailable on backorder
-
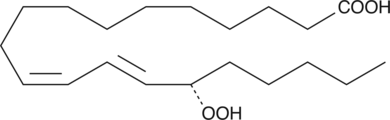
15(S)-HpETE is a monohydroperoxy polyunsaturated fatty acid (PUFA) produced by the action of 15-lipoxygenase (15-LO) on arachidonic acid. It is either metabolized to 14,15-leukotriene A4{391} or reduced to 15(S)-HETE by peroxidases.{3959,391} 15(S)-HpETE mediates a number of biological functions including the induction of c-fos and c-jun, and activation of AP-1.{4194} 15(S)-HpETE inhibits prostacyclin synthesis in porcine aortic microsomes and bovine endothelial cells, and can cause the suicide inactivation of porcine 12-LO.{3959,454,588}
Brand:CaymanSKU:44720 - 100 µgAvailable on backorder
-
15(S)-HpETE is a monohydroperoxy polyunsaturated fatty acid (PUFA) produced by the action of 15-lipoxygenase (15-LO) on arachidonic acid. It is either metabolized to 14,15-leukotriene A4{391} or reduced to 15(S)-HETE by peroxidases.{3959,391} 15(S)-HpETE mediates a number of biological functions including the induction of c-fos and c-jun, and activation of AP-1.{4194} 15(S)-HpETE inhibits prostacyclin synthesis in porcine aortic microsomes and bovine endothelial cells, and can cause the suicide inactivation of porcine 12-LO.{3959,454,588}
Brand:CaymanSKU:44720 - 25 µgAvailable on backorder
-
15(S)-HpETE is a monohydroperoxy polyunsaturated fatty acid (PUFA) produced by the action of 15-lipoxygenase (15-LO) on arachidonic acid. It is either metabolized to 14,15-leukotriene A4{391} or reduced to 15(S)-HETE by peroxidases.{3959,391} 15(S)-HpETE mediates a number of biological functions including the induction of c-fos and c-jun, and activation of AP-1.{4194} 15(S)-HpETE inhibits prostacyclin synthesis in porcine aortic microsomes and bovine endothelial cells, and can cause the suicide inactivation of porcine 12-LO.{3959,454,588}
Brand:CaymanSKU:44720 - 250 µgAvailable on backorder
-
15(S)-HpETE is a monohydroperoxy polyunsaturated fatty acid (PUFA) produced by the action of 15-lipoxygenase (15-LO) on arachidonic acid. It is either metabolized to 14,15-leukotriene A4{391} or reduced to 15(S)-HETE by peroxidases.{3959,391} 15(S)-HpETE mediates a number of biological functions including the induction of c-fos and c-jun, and activation of AP-1.{4194} 15(S)-HpETE inhibits prostacyclin synthesis in porcine aortic microsomes and bovine endothelial cells, and can cause the suicide inactivation of porcine 12-LO.{3959,454,588}
Brand:CaymanSKU:44720 - 50 µgAvailable on backorder
-
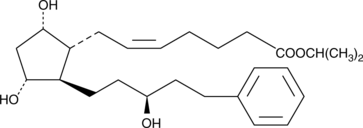
15(S)-Latanoprost is an analog of latanoprost in which the hydroxyl at carbon 15 is inverted relative to latanoprost. The IC50 values for the free acid forms of latanoprost and 15(S)-latanoprost were determined to be 3.6 nM and 24 nM, respectively, in a FP receptor binding assay using the cat iris sphincter muscle.{9736} A 3 µg dose of 15(S)-latanoprost caused a 1 mmHg reduction of IOP in normotensive cynomolgus monkeys.{9736} 15(S)-Latanoprost is a potential impurity in most commercial preparations of the latanoprost bulk drug product.
Brand:CaymanSKU:-Out of stock
-
15(S)-Latanoprost is an analog of latanoprost in which the hydroxyl at carbon 15 is inverted relative to latanoprost. The IC50 values for the free acid forms of latanoprost and 15(S)-latanoprost were determined to be 3.6 nM and 24 nM, respectively, in a FP receptor binding assay using the cat iris sphincter muscle.{9736} A 3 µg dose of 15(S)-latanoprost caused a 1 mmHg reduction of IOP in normotensive cynomolgus monkeys.{9736} 15(S)-Latanoprost is a potential impurity in most commercial preparations of the latanoprost bulk drug product.
Brand:CaymanSKU:-Out of stock
-
15(S)-Latanoprost is an analog of latanoprost in which the hydroxyl at carbon 15 is inverted relative to latanoprost. The IC50 values for the free acid forms of latanoprost and 15(S)-latanoprost were determined to be 3.6 nM and 24 nM, respectively, in a FP receptor binding assay using the cat iris sphincter muscle.{9736} A 3 µg dose of 15(S)-latanoprost caused a 1 mmHg reduction of IOP in normotensive cynomolgus monkeys.{9736} 15(S)-Latanoprost is a potential impurity in most commercial preparations of the latanoprost bulk drug product.
Brand:CaymanSKU:-Out of stock
-
15(S)-Latanoprost is an analog of latanoprost in which the hydroxyl at carbon 15 is inverted relative to latanoprost. The IC50 values for the free acid forms of latanoprost and 15(S)-latanoprost were determined to be 3.6 nM and 24 nM, respectively, in a FP receptor binding assay using the cat iris sphincter muscle.{9736} A 3 µg dose of 15(S)-latanoprost caused a 1 mmHg reduction of IOP in normotensive cynomolgus monkeys.{9736} 15(S)-Latanoprost is a potential impurity in most commercial preparations of the latanoprost bulk drug product.
Brand:CaymanSKU:-Out of stock
-
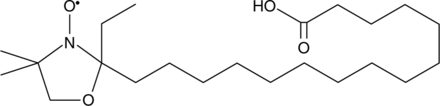
16-doxyl Stearic acid is a form of stearic acid (Item No. 10011298) that contains a 4,4-dimethyl-3-oxazolinyloxy (DOXYL) group, creating a hydrophobic spin label. It is commonly used to study molecular aspects of membranes and hydrophobic proteins.{48524,48525,48526}
Brand:CaymanSKU:20355 -Available on backorder
-
16-doxyl Stearic acid is a form of stearic acid (Item No. 10011298) that contains a 4,4-dimethyl-3-oxazolinyloxy (DOXYL) group, creating a hydrophobic spin label. It is commonly used to study molecular aspects of membranes and hydrophobic proteins.{48524,48525,48526}
Brand:CaymanSKU:20355 -Available on backorder
-
16-doxyl Stearic acid is a form of stearic acid (Item No. 10011298) that contains a 4,4-dimethyl-3-oxazolinyloxy (DOXYL) group, creating a hydrophobic spin label. It is commonly used to study molecular aspects of membranes and hydrophobic proteins.{48524,48525,48526}
Brand:CaymanSKU:20355 -Available on backorder
-
16-doxyl Stearic acid is a form of stearic acid (Item No. 10011298) that contains a 4,4-dimethyl-3-oxazolinyloxy (DOXYL) group, creating a hydrophobic spin label. It is commonly used to study molecular aspects of membranes and hydrophobic proteins.{48524,48525,48526}
Brand:CaymanSKU:20355 -Available on backorder
-
16-epi Latrunculin B, first isolated from the Red Sea sponge N. magnifica, is a stereoisomer of the actin polymerization inhibitor, latrunculin B (Item No. 10010631).{31273,31274} At 5-10 µg/ml, 16-epi latrunculin B can disrupt microfilament activity in an actin disruption assay.{31274} It also demonstrates cytotoxic activity in mouse KA31T and NIH3T3 tumor cells (GI50s = 1 and 4 µg/ml, respectively) and antiviral activity against herpes simplex type 1 virus (ED50 = 1 µg/ml).{31273}
Brand:CaymanSKU:19608 -Available on backorder
-
16-hydroxy Hexadecanoic acid is a metabolite of the saturated fatty acid palmitic acid (16:0) that has been hydroxylated on its terminal (ω) carbon. It is produced by ω-hydroxylation of palmitic acid by cytochrome P450 in both plants and animals.{19460,19462,19461,19458} In plants, it is commonly a component of cutin.{19459,19462}
Brand:CaymanSKU:9000789 - 1 gAvailable on backorder
-
16-hydroxy Hexadecanoic acid is a metabolite of the saturated fatty acid palmitic acid (16:0) that has been hydroxylated on its terminal (ω) carbon. It is produced by ω-hydroxylation of palmitic acid by cytochrome P450 in both plants and animals.{19460,19462,19461,19458} In plants, it is commonly a component of cutin.{19459,19462}
Brand:CaymanSKU:9000789 - 5 gAvailable on backorder
-
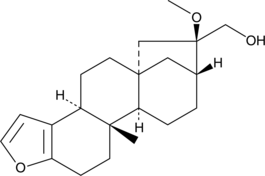
16-O-Methylcafestol is a diterpene that has been found in robusta (C. canephora) coffee beans.{42528} It has been used as a marker for robusta contamination and or adulteration of Arabica (C. arabica) roast coffee grounds in commercial production.
Brand:CaymanSKU:20259 -Available on backorder
-
16-O-Methylcafestol is a diterpene that has been found in robusta (C. canephora) coffee beans.{42528} It has been used as a marker for robusta contamination and or adulteration of Arabica (C. arabica) roast coffee grounds in commercial production.
Brand:CaymanSKU:20259 -Available on backorder
-
16-O-Methylcafestol is a diterpene that has been found in robusta (C. canephora) coffee beans.{42528} It has been used as a marker for robusta contamination and or adulteration of Arabica (C. arabica) roast coffee grounds in commercial production.
Brand:CaymanSKU:20259 -Available on backorder
-
16-Oxokahweol is a synthetic diterpene and derivative of kahweol (Item No. 14015).{53495} It increases cytosolic glutathione S-transferase (GST) activity and acid-soluble sulfhydryl levels in mouse liver and small bowel mucosa when administered at a dose of 10 µmol/animal once per day for three days.
Brand:CaymanSKU:30117 - 1 mgAvailable on backorder
-
16-Oxokahweol is a synthetic diterpene and derivative of kahweol (Item No. 14015).{53495} It increases cytosolic glutathione S-transferase (GST) activity and acid-soluble sulfhydryl levels in mouse liver and small bowel mucosa when administered at a dose of 10 µmol/animal once per day for three days.
Brand:CaymanSKU:30117 - 10 mgAvailable on backorder
-
16-Oxokahweol is a synthetic diterpene and derivative of kahweol (Item No. 14015).{53495} It increases cytosolic glutathione S-transferase (GST) activity and acid-soluble sulfhydryl levels in mouse liver and small bowel mucosa when administered at a dose of 10 µmol/animal once per day for three days.
Brand:CaymanSKU:30117 - 5 mgAvailable on backorder
-
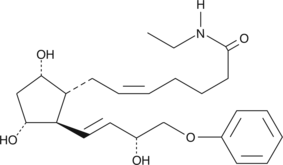
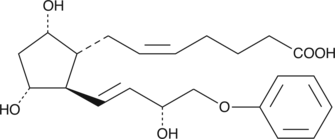
Prostaglandin F2α (PGF2α), acting through the FP receptor, causes smooth muscle contraction and exhibits potent luteolytic activity.{187,421,1651} 16-phenoxy PGF2α is a metabolically stable analog of PGF2α. It binds to the FP receptor on ovine luteal cells with much greater affinity (440%) than PGF2α.{2058} Ethyl amides of PGs serve as prodrugs, as they are hydrolyzed in certain tissues to generate the bioactive free acid.
Brand:CaymanSKU:10009875 - 1 mgAvailable on backorder
-
Prostaglandin F2α (PGF2α), acting through the FP receptor, causes smooth muscle contraction and exhibits potent luteolytic activity.{187,421,1651} 16-phenoxy PGF2α is a metabolically stable analog of PGF2α. It binds to the FP receptor on ovine luteal cells with much greater affinity (440%) than PGF2α.{2058} Ethyl amides of PGs serve as prodrugs, as they are hydrolyzed in certain tissues to generate the bioactive free acid.
Brand:CaymanSKU:10009875 - 10 mgAvailable on backorder
-
Prostaglandin F2α (PGF2α), acting through the FP receptor, causes smooth muscle contraction and exhibits potent luteolytic activity.{187,421,1651} 16-phenoxy PGF2α is a metabolically stable analog of PGF2α. It binds to the FP receptor on ovine luteal cells with much greater affinity (440%) than PGF2α.{2058} Ethyl amides of PGs serve as prodrugs, as they are hydrolyzed in certain tissues to generate the bioactive free acid.
Brand:CaymanSKU:10009875 - 5 mgAvailable on backorder
-
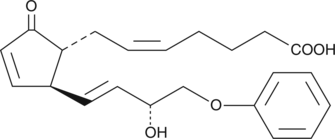
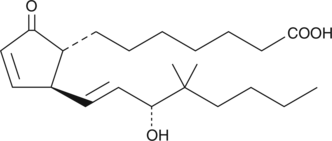
16-phenoxy tetranor PGA2 is a minor metabolite found in human plasma after intravenous administration of sulprostone.{3387} Its biological activity has not been studied or reported in the literature.
Brand:CaymanSKU:10285 - 1 mgAvailable on backorder
-
16-phenoxy tetranor PGA2 is a minor metabolite found in human plasma after intravenous administration of sulprostone.{3387} Its biological activity has not been studied or reported in the literature.
Brand:CaymanSKU:10285 - 10 mgAvailable on backorder
-
16-phenoxy tetranor PGA2 is a minor metabolite found in human plasma after intravenous administration of sulprostone.{3387} Its biological activity has not been studied or reported in the literature.
Brand:CaymanSKU:10285 - 5 mgAvailable on backorder
-
16-phenoxy tetranor PGE2 is the free acid form of sulprostone formed by the hydrolysis of the methylsulfonamide bond.{3387} 16-phenoxy tetranor PGE2 is a minor metabolite of sulprostone found in human plasma after parenteral administration of the drug.{3387}
Brand:CaymanSKU:- -
16-phenoxy tetranor PGE2 is the free acid form of sulprostone formed by the hydrolysis of the methylsulfonamide bond.{3387} 16-phenoxy tetranor PGE2 is a minor metabolite of sulprostone found in human plasma after parenteral administration of the drug.{3387}
Brand:CaymanSKU:- -
16-phenoxy tetranor PGE2 is the free acid form of sulprostone formed by the hydrolysis of the methylsulfonamide bond.{3387} 16-phenoxy tetranor PGE2 is a minor metabolite of sulprostone found in human plasma after parenteral administration of the drug.{3387}
Brand:CaymanSKU:- -
16-phenoxy PGF2α is a metabolically stable analog of PGF2α. It binds to the FP receptor on ovine luteal cells with much greater affinity (440%) than PGF2α.{2058}
Brand:CaymanSKU:-Out of stock
-
16-phenoxy PGF2α is a metabolically stable analog of PGF2α. It binds to the FP receptor on ovine luteal cells with much greater affinity (440%) than PGF2α.{2058}
Brand:CaymanSKU:-Out of stock
-
16-phenoxy PGF2α is a metabolically stable analog of PGF2α. It binds to the FP receptor on ovine luteal cells with much greater affinity (440%) than PGF2α.{2058}
Brand:CaymanSKU:-Out of stock
-
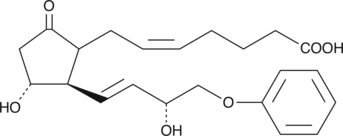
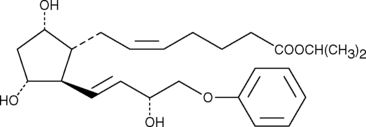
Prostaglandin F2α (PGF2α) drives luteolysis and smooth muscle contraction by activating the FP receptor. Stable, lipophilic analogs of PGF2α are used to modulate luteolysis and treat glaucoma. 16-phenoxy tetranor Prostaglandin F2α isopropyl ester (16-phenoxy tetranor PGF2α isopropyl ester) is a lipophilic analog of 16-phenoxy tetranor PGF2α. Isopropyl esters of PGs serve as prodrugs, as they are efficiently hydrolyzed in certain tissues to generate the bioactive free acid.
Brand:CaymanSKU:10010103 - 1 mgAvailable on backorder
-
Prostaglandin F2α (PGF2α) drives luteolysis and smooth muscle contraction by activating the FP receptor. Stable, lipophilic analogs of PGF2α are used to modulate luteolysis and treat glaucoma. 16-phenoxy tetranor Prostaglandin F2α isopropyl ester (16-phenoxy tetranor PGF2α isopropyl ester) is a lipophilic analog of 16-phenoxy tetranor PGF2α. Isopropyl esters of PGs serve as prodrugs, as they are efficiently hydrolyzed in certain tissues to generate the bioactive free acid.
Brand:CaymanSKU:10010103 - 10 mgAvailable on backorder
-
Prostaglandin F2α (PGF2α) drives luteolysis and smooth muscle contraction by activating the FP receptor. Stable, lipophilic analogs of PGF2α are used to modulate luteolysis and treat glaucoma. 16-phenoxy tetranor Prostaglandin F2α isopropyl ester (16-phenoxy tetranor PGF2α isopropyl ester) is a lipophilic analog of 16-phenoxy tetranor PGF2α. Isopropyl esters of PGs serve as prodrugs, as they are efficiently hydrolyzed in certain tissues to generate the bioactive free acid.
Brand:CaymanSKU:10010103 - 5 mgAvailable on backorder
-
Prostaglandin F2α (PGF2α) drives luteolysis and smooth muscle contraction by activating the FP receptor. Stable, lipophilic analogs of PGF2α are used to modulate luteolysis and treat glaucoma. 16-phenoxy tetranor Prostaglandin F2α isopropyl ester (16-phenoxy tetranor PGF2α isopropyl ester) is a lipophilic analog of 16-phenoxy tetranor PGF2α. Isopropyl esters of PGs serve as prodrugs, as they are efficiently hydrolyzed in certain tissues to generate the bioactive free acid.
Brand:CaymanSKU:10010103 - 500 µgAvailable on backorder
-
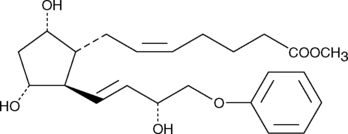
Prostaglandin F2α (PGF2α) drives luteolysis and smooth muscle contraction by activating the FP receptor. Stable, lipophilic analogs of PGF2α are used to modulate luteolysis and treat glaucoma. 16-phenoxy tetranor Prostaglandin F2α (16-phenoxy tetranor PGF2α) is a metabolically stable form of PGF2α containing a 16-phenoxy group at the ω-terminus. It binds to the FP receptor on ovine luteal cells with much greater affinity (440%) than PGF2α.{2058} 16-phenoxy tetranor PGF2α methyl ester is a lipophilic analog of 16-phenoxy tetranor PGF2α. Methyl esters of PGs serve as prodrugs, as they are efficiently hydrolyzed in certain tissues to generate the bioactive free acid.
Brand:CaymanSKU:10010102 - 1 mgAvailable on backorder
-
Prostaglandin F2α (PGF2α) drives luteolysis and smooth muscle contraction by activating the FP receptor. Stable, lipophilic analogs of PGF2α are used to modulate luteolysis and treat glaucoma. 16-phenoxy tetranor Prostaglandin F2α (16-phenoxy tetranor PGF2α) is a metabolically stable form of PGF2α containing a 16-phenoxy group at the ω-terminus. It binds to the FP receptor on ovine luteal cells with much greater affinity (440%) than PGF2α.{2058} 16-phenoxy tetranor PGF2α methyl ester is a lipophilic analog of 16-phenoxy tetranor PGF2α. Methyl esters of PGs serve as prodrugs, as they are efficiently hydrolyzed in certain tissues to generate the bioactive free acid.
Brand:CaymanSKU:10010102 - 5 mgAvailable on backorder
-
Prostaglandin F2α (PGF2α) drives luteolysis and smooth muscle contraction by activating the FP receptor. Stable, lipophilic analogs of PGF2α are used to modulate luteolysis and treat glaucoma. 16-phenoxy tetranor Prostaglandin F2α (16-phenoxy tetranor PGF2α) is a metabolically stable form of PGF2α containing a 16-phenoxy group at the ω-terminus. It binds to the FP receptor on ovine luteal cells with much greater affinity (440%) than PGF2α.{2058} 16-phenoxy tetranor PGF2α methyl ester is a lipophilic analog of 16-phenoxy tetranor PGF2α. Methyl esters of PGs serve as prodrugs, as they are efficiently hydrolyzed in certain tissues to generate the bioactive free acid.
Brand:CaymanSKU:10010102 - 500 µgAvailable on backorder
-
16,16-dimethyl PGA1 is a metabolism resistant analog of PGA1. In vitro, it inhibits the viral replication in both HSV and HIV-1 infection systems at concentrations that do not adversely alter cellular DNA synthesis. The ID50 for HSV-1 strains in Vero cells and human foreskin fibroblasts are 3.8-5.6 µg/ml and 4.6-7.3 µg/ml, respectively. The ID50 for T cells acutely infected with HIV-1 is 2.5 µg/ml.{811}
Brand:CaymanSKU:10080 - 1 mgAvailable on backorder
-
16,16-dimethyl PGA1 is a metabolism resistant analog of PGA1. In vitro, it inhibits the viral replication in both HSV and HIV-1 infection systems at concentrations that do not adversely alter cellular DNA synthesis. The ID50 for HSV-1 strains in Vero cells and human foreskin fibroblasts are 3.8-5.6 µg/ml and 4.6-7.3 µg/ml, respectively. The ID50 for T cells acutely infected with HIV-1 is 2.5 µg/ml.{811}
Brand:CaymanSKU:10080 - 10 mgAvailable on backorder
-
16,16-dimethyl PGA1 is a metabolism resistant analog of PGA1. In vitro, it inhibits the viral replication in both HSV and HIV-1 infection systems at concentrations that do not adversely alter cellular DNA synthesis. The ID50 for HSV-1 strains in Vero cells and human foreskin fibroblasts are 3.8-5.6 µg/ml and 4.6-7.3 µg/ml, respectively. The ID50 for T cells acutely infected with HIV-1 is 2.5 µg/ml.{811}
Brand:CaymanSKU:10080 - 5 mgAvailable on backorder
-
16,16-dimethyl PGA2 is a metabolism-resistant analog of PGA2 with a prolonged in vivo half-life. It inhibits the proliferation of Sendai virus in cultured African green monkey kidney cells by >90% at a concentration of 4 µg/ml.{2680} Daily infusion of 10 µg of 16,16-dimethyl PGA2 methyl ester into mice infected with influenza A virus increased survival by 40%.{1136} Similar treatment of mice inoculated with erythroleukemia cells delayed tumor growth and increased survival time.{1184}
Brand:CaymanSKU:10280 - 1 mgAvailable on backorder
-
16,16-dimethyl PGA2 is a metabolism-resistant analog of PGA2 with a prolonged in vivo half-life. It inhibits the proliferation of Sendai virus in cultured African green monkey kidney cells by >90% at a concentration of 4 µg/ml.{2680} Daily infusion of 10 µg of 16,16-dimethyl PGA2 methyl ester into mice infected with influenza A virus increased survival by 40%.{1136} Similar treatment of mice inoculated with erythroleukemia cells delayed tumor growth and increased survival time.{1184}
Brand:CaymanSKU:10280 - 10 mgAvailable on backorder
-
16,16-dimethyl PGA2 is a metabolism-resistant analog of PGA2 with a prolonged in vivo half-life. It inhibits the proliferation of Sendai virus in cultured African green monkey kidney cells by >90% at a concentration of 4 µg/ml.{2680} Daily infusion of 10 µg of 16,16-dimethyl PGA2 methyl ester into mice infected with influenza A virus increased survival by 40%.{1136} Similar treatment of mice inoculated with erythroleukemia cells delayed tumor growth and increased survival time.{1184}
Brand:CaymanSKU:10280 - 5 mgAvailable on backorder
-
16,16-dimethyl PGD2 is a metabolically stable synthetic analog of PGD2. It enhances ADP-induced human platelet aggregation and increases systemic blood pressure in rats.
Brand:CaymanSKU:12750 - 1 mgAvailable on backorder
-
16,16-dimethyl PGD2 is a metabolically stable synthetic analog of PGD2. It enhances ADP-induced human platelet aggregation and increases systemic blood pressure in rats.
Brand:CaymanSKU:12750 - 10 mgAvailable on backorder
-
16,16-dimethyl PGD2 is a metabolically stable synthetic analog of PGD2. It enhances ADP-induced human platelet aggregation and increases systemic blood pressure in rats.
Brand:CaymanSKU:12750 - 5 mgAvailable on backorder
-
16,16-dimethyl PGD2 is a metabolically stable synthetic analog of PGD2. It enhances ADP-induced human platelet aggregation and increases systemic blood pressure in rats.
Brand:CaymanSKU:12750 - 500 µgAvailable on backorder
-
16,16-dimethyl PGE1 is a metabolically stable synthetic analog of PGE1. It induces human vascular smooth muscle contractions in vitro. It is 2, 3, and 6 times more potent than PGF2α in contracting tracheal, bronchial, and bronchiolar smooth muscle, respectively.{2218}
Brand:CaymanSKU:- -
16,16-dimethyl PGE1 is a metabolically stable synthetic analog of PGE1. It induces human vascular smooth muscle contractions in vitro. It is 2, 3, and 6 times more potent than PGF2α in contracting tracheal, bronchial, and bronchiolar smooth muscle, respectively.{2218}
Brand:CaymanSKU:- -
16,16-dimethyl PGE1 is a metabolically stable synthetic analog of PGE1. It induces human vascular smooth muscle contractions in vitro. It is 2, 3, and 6 times more potent than PGF2α in contracting tracheal, bronchial, and bronchiolar smooth muscle, respectively.{2218}
Brand:CaymanSKU:- -
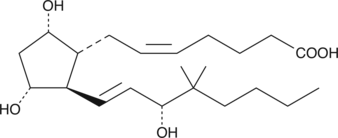
16,16-dimethyl PGE2 is a competitive inhibitor of 15-hydroxy PGDH, but it is not a substrate for the enzyme.{1789} Because of its resistance to metabolism by 15-hydroxy PGDH, it has a prolonged half-life in vivo. 16,16-dimethyl PGE2 acts as an agonist on most EP receptor subtypes, and has been used experimentally to induce cervical ripening, uterine contraction, and prevent ulceration of the gastric mucosa in rats and dogs.{437,1422} The Kd for activation of isolated EP2 receptors is about 1 nM.{1422} 16,16-dimethyl PGE2 can be used to preserve the self-renewal properties while preventing the differentiation of hematopoietic stem cells during expansion in culture.{27133,27095}
Brand:CaymanSKU:- -
16,16-dimethyl PGE2 is a competitive inhibitor of 15-hydroxy PGDH, but it is not a substrate for the enzyme.{1789} Because of its resistance to metabolism by 15-hydroxy PGDH, it has a prolonged half-life in vivo. 16,16-dimethyl PGE2 acts as an agonist on most EP receptor subtypes, and has been used experimentally to induce cervical ripening, uterine contraction, and prevent ulceration of the gastric mucosa in rats and dogs.{437,1422} The Kd for activation of isolated EP2 receptors is about 1 nM.{1422} 16,16-dimethyl PGE2 can be used to preserve the self-renewal properties while preventing the differentiation of hematopoietic stem cells during expansion in culture.{27133,27095}
Brand:CaymanSKU:- -
16,16-dimethyl PGE2 is a competitive inhibitor of 15-hydroxy PGDH, but it is not a substrate for the enzyme.{1789} Because of its resistance to metabolism by 15-hydroxy PGDH, it has a prolonged half-life in vivo. 16,16-dimethyl PGE2 acts as an agonist on most EP receptor subtypes, and has been used experimentally to induce cervical ripening, uterine contraction, and prevent ulceration of the gastric mucosa in rats and dogs.{437,1422} The Kd for activation of isolated EP2 receptors is about 1 nM.{1422} 16,16-dimethyl PGE2 can be used to preserve the self-renewal properties while preventing the differentiation of hematopoietic stem cells during expansion in culture.{27133,27095}
Brand:CaymanSKU:- -
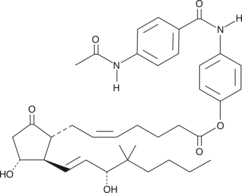
The p-(p-acetamidobenzamido) phenyl ester is a crystalline derivative of 16,16-dimethyl PGE2 and a potential prodrug.
Brand:CaymanSKU:- -
The p-(p-acetamidobenzamido) phenyl ester is a crystalline derivative of 16,16-dimethyl PGE2 and a potential prodrug.
Brand:CaymanSKU:- -
The p-(p-acetamidobenzamido) phenyl ester is a crystalline derivative of 16,16-dimethyl PGE2 and a potential prodrug.
Brand:CaymanSKU:- -
The p-(p-acetamidobenzamido) phenyl ester is a crystalline derivative of 16,16-dimethyl PGE2 and a potential prodrug.
Brand:CaymanSKU:- -
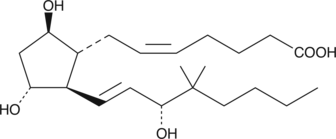
16,16-dimethyl PGF2α is a metabolically stable analog of PGF2α. It binds to the FP receptor on ovine luteal cells with slightly better affinity (159%) than PGF2α.{2058}
Brand:CaymanSKU:-Out of stock
-
16,16-dimethyl PGF2α is a metabolically stable analog of PGF2α. It binds to the FP receptor on ovine luteal cells with slightly better affinity (159%) than PGF2α.{2058}
Brand:CaymanSKU:-Out of stock
-
16,16-dimethyl PGF2α is a metabolically stable analog of PGF2α. It binds to the FP receptor on ovine luteal cells with slightly better affinity (159%) than PGF2α.{2058}
Brand:CaymanSKU:-Out of stock
-
16,16-dimethyl PGF2β is a metabolically stable analog of PGF2β. It prevents bronchospasm in asthmatics but is less potent than PGE2.{6596}
Brand:CaymanSKU:-Out of stock
-
16,16-dimethyl PGF2β is a metabolically stable analog of PGF2β. It prevents bronchospasm in asthmatics but is less potent than PGE2.{6596}
Brand:CaymanSKU:-Out of stock
-
16,16-dimethyl PGF2β is a metabolically stable analog of PGF2β. It prevents bronchospasm in asthmatics but is less potent than PGE2.{6596}
Brand:CaymanSKU:-Out of stock
-
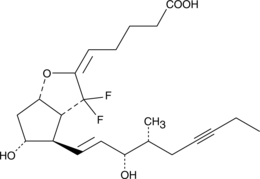
Prostaglandin I2 is an unstable prostanoid which, through the ‘I prostanoid’ (IP) receptor, inhibits platelet aggregation and promotes vasodilatation in pulmonary vascular beds. AFP 07 is a 7,7-difluoroprostacyclin derivative that acts as a selective and highly potent agonist for the IP receptor (Ki = 0.561 nM).{7236} AFP 07 shows weaker affinity for EP receptors, with Ki values > 100 nM for EP1-3 and > 10 nM for EP4.{7236,15488} 16(R)-AFP 07 is an epimer of AFP 07. Its biological properties, particularly through the IP and EP receptors, remain to be evaluated.
Brand:CaymanSKU:10991 - 1 mgAvailable on backorder
-
Prostaglandin I2 is an unstable prostanoid which, through the ‘I prostanoid’ (IP) receptor, inhibits platelet aggregation and promotes vasodilatation in pulmonary vascular beds. AFP 07 is a 7,7-difluoroprostacyclin derivative that acts as a selective and highly potent agonist for the IP receptor (Ki = 0.561 nM).{7236} AFP 07 shows weaker affinity for EP receptors, with Ki values > 100 nM for EP1-3 and > 10 nM for EP4.{7236,15488} 16(R)-AFP 07 is an epimer of AFP 07. Its biological properties, particularly through the IP and EP receptors, remain to be evaluated.
Brand:CaymanSKU:10991 - 5 mgAvailable on backorder
-
Prostaglandin I2 is an unstable prostanoid which, through the ‘I prostanoid’ (IP) receptor, inhibits platelet aggregation and promotes vasodilatation in pulmonary vascular beds. AFP 07 is a 7,7-difluoroprostacyclin derivative that acts as a selective and highly potent agonist for the IP receptor (Ki = 0.561 nM).{7236} AFP 07 shows weaker affinity for EP receptors, with Ki values > 100 nM for EP1-3 and > 10 nM for EP4.{7236,15488} 16(R)-AFP 07 is an epimer of AFP 07. Its biological properties, particularly through the IP and EP receptors, remain to be evaluated.
Brand:CaymanSKU:10991 - 500 µgAvailable on backorder
-
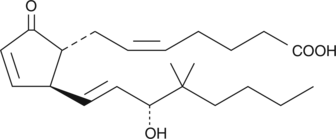
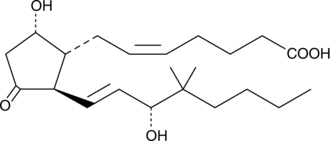
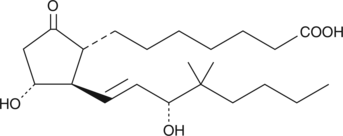
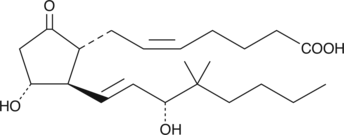
Electrolyte and fluid transport in the kidney are regulated in part by arachidonic acid and its metabolites. 16-HETE is a minor CYP450 metabolite of arachidonic acid released by the kidney upon angiotensin II stimulation that demonstrates stereospecific biological activity. Electrolyte and fluid transport in the kidney are regulated in part by arachidonic acid and its metabolites. 16-HETE is a minor CYP450 metabolite of arachidonic acid released by the kidney upon angiotensin II stimulation that demonstrates stereospecific biological activity. 16(S)-HETE inhibits proximal tubule ATPase activity by as much as 60% at a concentration of 2 µM.{3825}
Brand:CaymanSKU:10004385 - 100 µgAvailable on backorder
-
Electrolyte and fluid transport in the kidney are regulated in part by arachidonic acid and its metabolites. 16-HETE is a minor CYP450 metabolite of arachidonic acid released by the kidney upon angiotensin II stimulation that demonstrates stereospecific biological activity. Electrolyte and fluid transport in the kidney are regulated in part by arachidonic acid and its metabolites. 16-HETE is a minor CYP450 metabolite of arachidonic acid released by the kidney upon angiotensin II stimulation that demonstrates stereospecific biological activity. 16(S)-HETE inhibits proximal tubule ATPase activity by as much as 60% at a concentration of 2 µM.{3825}
Brand:CaymanSKU:10004385 - 25 µgAvailable on backorder
-
Electrolyte and fluid transport in the kidney are regulated in part by arachidonic acid and its metabolites. 16-HETE is a minor CYP450 metabolite of arachidonic acid released by the kidney upon angiotensin II stimulation that demonstrates stereospecific biological activity. Electrolyte and fluid transport in the kidney are regulated in part by arachidonic acid and its metabolites. 16-HETE is a minor CYP450 metabolite of arachidonic acid released by the kidney upon angiotensin II stimulation that demonstrates stereospecific biological activity. 16(S)-HETE inhibits proximal tubule ATPase activity by as much as 60% at a concentration of 2 µM.{3825}
Brand:CaymanSKU:10004385 - 50 µgAvailable on backorder
-
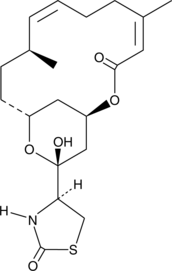
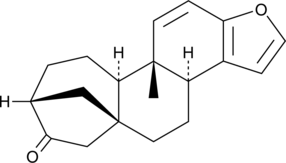
Iloprost is a second generation structural analog of prostacyclin (PGI2) with about ten-fold greater potency than the first generation stable analogs, typified by carbaprostacyclin.{3347} Iloprost binds with equal affinity to the recombinant human IP and EP1 receptors with a Ki value of 11 nM.{8322} Most preparations of iloprost contain 16(S) and 16(R) stereoisomers. 16(R)-Iloprost inhibits platelet aggregation with an IC50 value of 65 nM.{16394}
Brand:CaymanSKU:- -
Iloprost is a second generation structural analog of prostacyclin (PGI2) with about ten-fold greater potency than the first generation stable analogs, typified by carbaprostacyclin.{3347} Iloprost binds with equal affinity to the recombinant human IP and EP1 receptors with a Ki value of 11 nM.{8322} Most preparations of iloprost contain 16(S) and 16(R) stereoisomers. 16(R)-Iloprost inhibits platelet aggregation with an IC50 value of 65 nM.{16394}
Brand:CaymanSKU:- -
Iloprost is a second generation structural analog of prostacyclin (PGI2) with about ten-fold greater potency than the first generation stable analogs, typified by carbaprostacyclin.{3347} Iloprost binds with equal affinity to the recombinant human IP and EP1 receptors with a Ki value of 11 nM.{8322} Most preparations of iloprost contain 16(S) and 16(R) stereoisomers. 16(R)-Iloprost inhibits platelet aggregation with an IC50 value of 65 nM.{16394}
Brand:CaymanSKU:- -
Electrolyte and fluid transport in the kidney are regulated in part by arachidonic acid and its metabolites. 16-HETE is a minor CYP450 metabolite of arachidonic acid released by the kidney upon angiotensin II stimulation that demonstrates stereospecific biological activity. 16(S)-HETE inhibits proximal tubule ATPase activity by as much as 60% at a concentration of 2 µM.{3825}
Brand:CaymanSKU:10004384 - 100 µgAvailable on backorder
-
Electrolyte and fluid transport in the kidney are regulated in part by arachidonic acid and its metabolites. 16-HETE is a minor CYP450 metabolite of arachidonic acid released by the kidney upon angiotensin II stimulation that demonstrates stereospecific biological activity. 16(S)-HETE inhibits proximal tubule ATPase activity by as much as 60% at a concentration of 2 µM.{3825}
Brand:CaymanSKU:10004384 - 25 µgAvailable on backorder
-
Electrolyte and fluid transport in the kidney are regulated in part by arachidonic acid and its metabolites. 16-HETE is a minor CYP450 metabolite of arachidonic acid released by the kidney upon angiotensin II stimulation that demonstrates stereospecific biological activity. 16(S)-HETE inhibits proximal tubule ATPase activity by as much as 60% at a concentration of 2 µM.{3825}
Brand:CaymanSKU:10004384 - 50 µgAvailable on backorder
-
Iloprost is a second generation structural analog of prostacyclin (PGI2) with about ten-fold greater potency than the first generation stable analogs, typified by carbaprostacyclin.{3347} Iloprost binds with equal affinity to the recombinant human IP and EP1 receptors with a Ki value of 11 nM.{8322} Most preparations of iloprost contain 16(S) and 16(R) stereoisomers. 16(S)-Iloprost potently inhibits platelet aggregation with an IC50 value of 3.5 nM.{16394}
Brand:CaymanSKU:- -
Iloprost is a second generation structural analog of prostacyclin (PGI2) with about ten-fold greater potency than the first generation stable analogs, typified by carbaprostacyclin.{3347} Iloprost binds with equal affinity to the recombinant human IP and EP1 receptors with a Ki value of 11 nM.{8322} Most preparations of iloprost contain 16(S) and 16(R) stereoisomers. 16(S)-Iloprost potently inhibits platelet aggregation with an IC50 value of 3.5 nM.{16394}
Brand:CaymanSKU:- -
Iloprost is a second generation structural analog of prostacyclin (PGI2) with about ten-fold greater potency than the first generation stable analogs, typified by carbaprostacyclin.{3347} Iloprost binds with equal affinity to the recombinant human IP and EP1 receptors with a Ki value of 11 nM.{8322} Most preparations of iloprost contain 16(S) and 16(R) stereoisomers. 16(S)-Iloprost potently inhibits platelet aggregation with an IC50 value of 3.5 nM.{16394}
Brand:CaymanSKU:- -
16F16 is a protein disulfide isomerase (PDI) inhibitor.{54267} It inhibits PDI reductase activity in an enzyme assay when used at concentrations ranging from 1 to 100 µg/ml.{54267} 16F16 reduces PC12 cell apoptosis induced by the misfolded huntingtin protein HTTQ103. It suppresses PDI-dependent mitochondrial outer membrane permeabilization (MOMP) in isolated PC12 cell mitochondria. 16F16 (2, 3, 4, and 10 µM) reduces HTTN90Q73 mutant huntingtin-induced medium spinal neuron death and MOMP in rat corticostriatal slices. It also reduces pyramidal neuron death induced by amyloid-β precursor protein (APP) in rat corticostriatal slices.
Brand:CaymanSKU:-Available on backorder
-
16F16 is a protein disulfide isomerase (PDI) inhibitor.{54267} It inhibits PDI reductase activity in an enzyme assay when used at concentrations ranging from 1 to 100 µg/ml.{54267} 16F16 reduces PC12 cell apoptosis induced by the misfolded huntingtin protein HTTQ103. It suppresses PDI-dependent mitochondrial outer membrane permeabilization (MOMP) in isolated PC12 cell mitochondria. 16F16 (2, 3, 4, and 10 µM) reduces HTTN90Q73 mutant huntingtin-induced medium spinal neuron death and MOMP in rat corticostriatal slices. It also reduces pyramidal neuron death induced by amyloid-β precursor protein (APP) in rat corticostriatal slices.
Brand:CaymanSKU:-Available on backorder
-
16F16 is a protein disulfide isomerase (PDI) inhibitor.{54267} It inhibits PDI reductase activity in an enzyme assay when used at concentrations ranging from 1 to 100 µg/ml.{54267} 16F16 reduces PC12 cell apoptosis induced by the misfolded huntingtin protein HTTQ103. It suppresses PDI-dependent mitochondrial outer membrane permeabilization (MOMP) in isolated PC12 cell mitochondria. 16F16 (2, 3, 4, and 10 µM) reduces HTTN90Q73 mutant huntingtin-induced medium spinal neuron death and MOMP in rat corticostriatal slices. It also reduces pyramidal neuron death induced by amyloid-β precursor protein (APP) in rat corticostriatal slices.
Brand:CaymanSKU:-Available on backorder
-
16F16 is a protein disulfide isomerase (PDI) inhibitor.{54267} It inhibits PDI reductase activity in an enzyme assay when used at concentrations ranging from 1 to 100 µg/ml.{54267} 16F16 reduces PC12 cell apoptosis induced by the misfolded huntingtin protein HTTQ103. It suppresses PDI-dependent mitochondrial outer membrane permeabilization (MOMP) in isolated PC12 cell mitochondria. 16F16 (2, 3, 4, and 10 µM) reduces HTTN90Q73 mutant huntingtin-induced medium spinal neuron death and MOMP in rat corticostriatal slices. It also reduces pyramidal neuron death induced by amyloid-β precursor protein (APP) in rat corticostriatal slices.
Brand:CaymanSKU:-Available on backorder
-
The naturally-occurring estrogens are estrone (E1, Item No. 10006485), estradiol (E2, Item No. 10006315), and estriol (E3, Item No. 10006484).{9611} 16α-hydroxy Estrone (16α-OHE1) is a hydroxylated metabolite of E1 as well as an interconversion product with E2.{24474,24473} E1 is 16α-hydroxylated by cytochrome P450 (CYP) isoforms, including CYP1A1, CYP3A5, CYP3A4, and CYP3A7, with CYP3A5 being breast-specific.{24483} 16α-OHE1 is sulphatized or glucuronidated before excretion.{24483} It is increased in rheumatoid arthritis and decreased by physical activity.{24474,24473} Unlike the parent estrogens and other hydroxylated metabolites of E1, 16α-OHE1 binds covalently and persistently activates estrogen receptors.{24475} In addition, this metabolite increases cell proliferation and does not suppress TNF-α secretion, whereas other estrogen metabolites are not pro-proliferative and have marked effects on TNF-α secretion.{24474,24483} The levels of 16α-OHE1 are increased in some forms of hormone therapy.{24472} Because hormone therapy increases breast cancer risk, 16α-OHE1 has been implicated as a risk factor for breast cancer, although supportive data remains elusive.{24476,24483,24472}
Brand:CaymanSKU:- -
The naturally-occurring estrogens are estrone (E1, Item No. 10006485), estradiol (E2, Item No. 10006315), and estriol (E3, Item No. 10006484).{9611} 16α-hydroxy Estrone (16α-OHE1) is a hydroxylated metabolite of E1 as well as an interconversion product with E2.{24474,24473} E1 is 16α-hydroxylated by cytochrome P450 (CYP) isoforms, including CYP1A1, CYP3A5, CYP3A4, and CYP3A7, with CYP3A5 being breast-specific.{24483} 16α-OHE1 is sulphatized or glucuronidated before excretion.{24483} It is increased in rheumatoid arthritis and decreased by physical activity.{24474,24473} Unlike the parent estrogens and other hydroxylated metabolites of E1, 16α-OHE1 binds covalently and persistently activates estrogen receptors.{24475} In addition, this metabolite increases cell proliferation and does not suppress TNF-α secretion, whereas other estrogen metabolites are not pro-proliferative and have marked effects on TNF-α secretion.{24474,24483} The levels of 16α-OHE1 are increased in some forms of hormone therapy.{24472} Because hormone therapy increases breast cancer risk, 16α-OHE1 has been implicated as a risk factor for breast cancer, although supportive data remains elusive.{24476,24483,24472}
Brand:CaymanSKU:- -
The naturally-occurring estrogens are estrone (E1, Item No. 10006485), estradiol (E2, Item No. 10006315), and estriol (E3, Item No. 10006484).{9611} 16α-hydroxy Estrone (16α-OHE1) is a hydroxylated metabolite of E1 as well as an interconversion product with E2.{24474,24473} E1 is 16α-hydroxylated by cytochrome P450 (CYP) isoforms, including CYP1A1, CYP3A5, CYP3A4, and CYP3A7, with CYP3A5 being breast-specific.{24483} 16α-OHE1 is sulphatized or glucuronidated before excretion.{24483} It is increased in rheumatoid arthritis and decreased by physical activity.{24474,24473} Unlike the parent estrogens and other hydroxylated metabolites of E1, 16α-OHE1 binds covalently and persistently activates estrogen receptors.{24475} In addition, this metabolite increases cell proliferation and does not suppress TNF-α secretion, whereas other estrogen metabolites are not pro-proliferative and have marked effects on TNF-α secretion.{24474,24483} The levels of 16α-OHE1 are increased in some forms of hormone therapy.{24472} Because hormone therapy increases breast cancer risk, 16α-OHE1 has been implicated as a risk factor for breast cancer, although supportive data remains elusive.{24476,24483,24472}
Brand:CaymanSKU:- -
16α-hydroxy Prednisolone is a stereoselective metabolite of the 22(R) epimer of the glucocorticoid budesonide.{43877,43876} It is formed via metabolism of 22(R)-budesonide by the cytochrome P450 (CYP) isoform CYP3A.{43876}
Brand:CaymanSKU:27169 - 1 gAvailable on backorder
-
16α-hydroxy Prednisolone is a stereoselective metabolite of the 22(R) epimer of the glucocorticoid budesonide.{43877,43876} It is formed via metabolism of 22(R)-budesonide by the cytochrome P450 (CYP) isoform CYP3A.{43876}
Brand:CaymanSKU:27169 - 10 gAvailable on backorder
-
16α-hydroxy Prednisolone is a stereoselective metabolite of the 22(R) epimer of the glucocorticoid budesonide.{43877,43876} It is formed via metabolism of 22(R)-budesonide by the cytochrome P450 (CYP) isoform CYP3A.{43876}
Brand:CaymanSKU:27169 - 25 gAvailable on backorder
-
16α-hydroxy Prednisolone is a stereoselective metabolite of the 22(R) epimer of the glucocorticoid budesonide.{43877,43876} It is formed via metabolism of 22(R)-budesonide by the cytochrome P450 (CYP) isoform CYP3A.{43876}
Brand:CaymanSKU:27169 - 5 gAvailable on backorder
-
16α-Hydroxyetiocholanolone is a metabolite of 16α-hydroxydehydroisoandrosterone (16α-DHEA) and androstenedione.{52423,52424}
Brand:CaymanSKU:27683 - 1 mgAvailable on backorder
-
16α-Hydroxyetiocholanolone is a metabolite of 16α-hydroxydehydroisoandrosterone (16α-DHEA) and androstenedione.{52423,52424}
Brand:CaymanSKU:27683 - 10 mgAvailable on backorder
-
16α-Hydroxyetiocholanolone is a metabolite of 16α-hydroxydehydroisoandrosterone (16α-DHEA) and androstenedione.{52423,52424}
Brand:CaymanSKU:27683 - 5 mgAvailable on backorder
-
17-AAG is an inhibitor of heat shock protein 90 (Hsp90) and a derivative of geldanamycin (Item No. 13355) that selectively inhibits BT474 tumor cell Hsp90 over fibroblast Hsp90 (IC50s = 5 and 943 nM, respectively).{46058} It inhibits the growth of prostate cancer cell lines (IC50s = 25-45 nM) and promotes the degradation of HER2 and induces growth arrest and apoptosis in breast cancer cells overexpressing HER2 (IC50s = 4-72 nM).{20728,20725} In vivo, 17-AAG (25 mg/kg, i.p.) reduces tumor size in a G-415 gallbladder cancer mouse xenograft model.{46059}
Brand:CaymanSKU:11039 - 1 mgAvailable on backorder
-
17-AAG is an inhibitor of heat shock protein 90 (Hsp90) and a derivative of geldanamycin (Item No. 13355) that selectively inhibits BT474 tumor cell Hsp90 over fibroblast Hsp90 (IC50s = 5 and 943 nM, respectively).{46058} It inhibits the growth of prostate cancer cell lines (IC50s = 25-45 nM) and promotes the degradation of HER2 and induces growth arrest and apoptosis in breast cancer cells overexpressing HER2 (IC50s = 4-72 nM).{20728,20725} In vivo, 17-AAG (25 mg/kg, i.p.) reduces tumor size in a G-415 gallbladder cancer mouse xenograft model.{46059}
Brand:CaymanSKU:11039 - 10 mgAvailable on backorder
-
17-AAG is an inhibitor of heat shock protein 90 (Hsp90) and a derivative of geldanamycin (Item No. 13355) that selectively inhibits BT474 tumor cell Hsp90 over fibroblast Hsp90 (IC50s = 5 and 943 nM, respectively).{46058} It inhibits the growth of prostate cancer cell lines (IC50s = 25-45 nM) and promotes the degradation of HER2 and induces growth arrest and apoptosis in breast cancer cells overexpressing HER2 (IC50s = 4-72 nM).{20728,20725} In vivo, 17-AAG (25 mg/kg, i.p.) reduces tumor size in a G-415 gallbladder cancer mouse xenograft model.{46059}
Brand:CaymanSKU:11039 - 5 mgAvailable on backorder
-
Geldanamycin (Item No. 13355) is a potent inhibitor of Hsp90 that has poor water solubility. 17-DMAG is a water-soluble derivative of geldanamycin which potently inhibits Hsp90 (IC50 = 24 nM) and has excellent bioavailability and tissue distribution in animals.{20709} Like other Hsp90 inhibitors, 17-DMAG has diverse anti-tumor actions and has potential in treating certain types of cancer.{20708,18248,20710,20713,20711} This compound also suppresses inflammation by interfering with signaling through the NF-κB pathway.{20712,20714} 17-DMAG also ameliorates high fat diet-induced renal failure in a mouse model of diabetes.{20715}
Brand:CaymanSKU:11036 - 1 mgAvailable on backorder
-
Geldanamycin (Item No. 13355) is a potent inhibitor of Hsp90 that has poor water solubility. 17-DMAG is a water-soluble derivative of geldanamycin which potently inhibits Hsp90 (IC50 = 24 nM) and has excellent bioavailability and tissue distribution in animals.{20709} Like other Hsp90 inhibitors, 17-DMAG has diverse anti-tumor actions and has potential in treating certain types of cancer.{20708,18248,20710,20713,20711} This compound also suppresses inflammation by interfering with signaling through the NF-κB pathway.{20712,20714} 17-DMAG also ameliorates high fat diet-induced renal failure in a mouse model of diabetes.{20715}
Brand:CaymanSKU:11036 - 10 mgAvailable on backorder
-
Geldanamycin (Item No. 13355) is a potent inhibitor of Hsp90 that has poor water solubility. 17-DMAG is a water-soluble derivative of geldanamycin which potently inhibits Hsp90 (IC50 = 24 nM) and has excellent bioavailability and tissue distribution in animals.{20709} Like other Hsp90 inhibitors, 17-DMAG has diverse anti-tumor actions and has potential in treating certain types of cancer.{20708,18248,20710,20713,20711} This compound also suppresses inflammation by interfering with signaling through the NF-κB pathway.{20712,20714} 17-DMAG also ameliorates high fat diet-induced renal failure in a mouse model of diabetes.{20715}
Brand:CaymanSKU:11036 - 5 mgAvailable on backorder
-
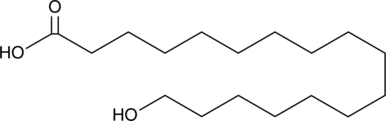
17-hydroxy Heptadecanoic acid is a hydroxylated fatty acid that has been found in aeolian particles collected on the Frioul Islands and in the outer bark of E. globulus.{38891,38892} It has been used in the synthesis of various macrocyclic lactones.{38893} [Matreya, LLC. Catalog No. 1760]
Brand:CaymanSKU:24650 - 25 mgAvailable on backorder
-
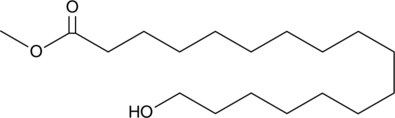
17-hydroxy Heptadecanoic acid methyl ester is a hydroxylated fatty acid methyl ester and substrate for lactonizing lipase from Pseudomonas nov. sp. 109, a lipase that catalyzes the synthesis of macrocyclic lactones.{39664} [Matreya, LLC. Catalog No. 1761]
Brand:CaymanSKU:24651 - 25 mgAvailable on backorder
-
17-hydroxy Venturicidin A is a macrolide fungal metabolite originally isolated from Streptomyces.{49095} It has antibiotic activity against M. luteus, B. subtilis, and S. aureus and antifungal activity against V. dahlia, Fusarium, and C. tropicalis in a disc assay.
Brand:CaymanSKU:27508 - 1 mgAvailable on backorder
-
17-hydroxy Venturicidin A is a macrolide fungal metabolite originally isolated from Streptomyces.{49095} It has antibiotic activity against M. luteus, B. subtilis, and S. aureus and antifungal activity against V. dahlia, Fusarium, and C. tropicalis in a disc assay.
Brand:CaymanSKU:27508 - 500 µgAvailable on backorder
-
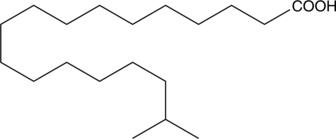
17-methyl Stearic acid is a methyl branched-chain fatty acid that has been found in murine meibomian glands, C. cornucopioides mushrooms, and Phyllobacterium species.{42640,42641,42642} It has also been found in the aerial parts of C. ladanifer and its concentration exhibits seasonal variation.{42643}
Brand:CaymanSKU:26766 - 1 mgAvailable on backorder
-
17-methyl Stearic acid is a methyl branched-chain fatty acid that has been found in murine meibomian glands, C. cornucopioides mushrooms, and Phyllobacterium species.{42640,42641,42642} It has also been found in the aerial parts of C. ladanifer and its concentration exhibits seasonal variation.{42643}
Brand:CaymanSKU:26766 - 10 mgAvailable on backorder
-
17-methyl Stearic acid is a methyl branched-chain fatty acid that has been found in murine meibomian glands, C. cornucopioides mushrooms, and Phyllobacterium species.{42640,42641,42642} It has also been found in the aerial parts of C. ladanifer and its concentration exhibits seasonal variation.{42643}
Brand:CaymanSKU:26766 - 5 mgAvailable on backorder