Cayman
Showing 24151–24300 of 45550 results
-
Immunogen: synthetic peptide from the C-terminal region of human HSL receptor • Species Reactivity: Human, mouse, rat HSL receptor • Host: rabbit • Application: WB
Brand:CaymanSKU:10006371- 500 µlAvailable on backorder
Immunogen: synthetic peptide from the C-terminal region of human HSL receptor • Species Reactivity: Human, mouse, rat HSL receptor • Host: rabbit • Application: WB
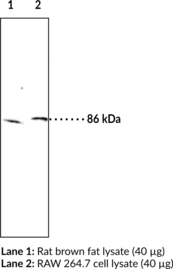
Brand:CaymanSKU:10006371- 500 µlHormone-sensitive lipase (HSL) catalyzes the hydrolysis of tri-, di-, and monoacylglycerols, as well as cholesterol esters and thus mobilizes fatty acid and provides a primary source of energy in mammals.{13168} The enzyme is highly expressed in adipose tissue and steroidogenic tissues, and less abundantly in skeletal muscle, heart, brain, pancreatic beta cells, adrenal gland, ovaries, testes, and macrophages. Its presence in various tissues indicates the enzyme plays diverse roles including those in steroidogenesis and spermatogenesis, foam cell formation in atherosclerosis, and diabetic pathology.{13168,13167} Human HSL cDNA encodes a 775 amino acid protein with an estimated molecular size of 84 kDa.{13169} A second, larger isoform encoded by a unique testis mRNA was later identified.{13171,13166} Cayman’s HSL Polyclonal Antibody can be used for Western blot applications. The antibody recognizes HSL at 86 kDa from human, mouse, and rat samples.
Brand:CaymanSKU:10006371 - 500 µlAvailable on backorder
Impurity assessment is a key step during the drug development production of recombinant proteins, including therapeutic proteins. Specific impurities coming from the cells mediating the protein expression, known as ‘Host Cell Proteins (HCP)’, are generated and need to be removed. This kit is intended for use in assessing relative quantities of E. coli HCP in manufactured or research bioproducts. Polyclonal antibodies used in this kit have been generated against several strains of E. coli and specifically selected for their recognition of a large spectrum of E. coli proteins. Thus, this kit can be considered as generic and allows a relative-quantitative determination of E. coli HCP in many types of samples, such as samples issued from the purification process (HCP clearance), process control, quality control, or product release. Using this kit, HCP concentration is measured in ng/ml (HCP equivalent is extrapolated from a standard curve). Conventionally, the HCP content in a product will finally be expressed in ng/mg, where ng represents HCP mass and mg represents the product mass. Note that contrary to the concentration measurement of the product, the HCP signal is only reflective of antibody binding and does not strictly reflect the mass of HCP. This kit has been successfully validated for recovery and precision using reconstituted HCP samples and tested against different final products. Given the diversity of final products, all potential matrix effects cannot be known and it is recommended that you test the suitability of the kit for use with your own HCP samples in your laboratory. This kit should be used as one part of your complete HCP analysis. [Bertin Catalog No. A05034]
Brand:CaymanSKU:18919 - 96 wellsAvailable on backorder
HPA-12 is a ceramide analog and inhibitor of ceramide transfer protein (CERT).{52022,52023} It inhibits binding of a ceramide probe to the isolated steroidogenic acute regulatory protein-related lipid transfer (START) domain of CERT with an EC50 value of 4 μM in a TR-FRET assay.{52023} HPA-12 (2.5 μM) inhibits conversion of the fluorescent ceramide analog C5-DMB-Cer to C5-DMB-sphingomyelin in CHO and HeLa cells.{52022} It also inhibits sphingomyelin synthesis in wild-type, but not ATP-dependent ceramide transport-deficient, CHO cells when used at a concentration of 1 μM.
Brand:CaymanSKU:28350 - 5 mgAvailable on backorder
The biology of highly reactive oxygen radical species is of great interest in many biomedical research disciplines, including neurodegeneration, aging, cancer, and infectious diseases.{9477} There are a number of fluorescent reagents, such as 2,7-dichlorodihydrofluorescein (DCDHF), that can be used to detect free radicals, but they have significant limitations due to their facile oxidation by light and numerous non-radical oxidants such as hydrogen peroxide (H2O2).{12088} HPF is a cell-permeable aromatic amino-fluorescein derivative that has little intrinsic fluorescence.{11242} It undergoes oxidation only by highly reactive oxygen species (hROS) such as the hydroxyl radical, peroxynitrite, and hROS generated from a peroxidase/H2O2 system. It is inert to hypochlorite ion, nitric oxide, hydrogen peroxide (H2O2), superoxide, and other oxidants. Upon oxidation, HPF is converted to the highly fluorescent molecule fluorescein, with excitation/emission maxima of 490/515 nm, respectively, allowing the simple direct detection of highly reactive biological radicals.
Brand:CaymanSKU:10159 - 1 mgAvailable on backorder
The biology of highly reactive oxygen radical species is of great interest in many biomedical research disciplines, including neurodegeneration, aging, cancer, and infectious diseases.{9477} There are a number of fluorescent reagents, such as 2,7-dichlorodihydrofluorescein (DCDHF), that can be used to detect free radicals, but they have significant limitations due to their facile oxidation by light and numerous non-radical oxidants such as hydrogen peroxide (H2O2).{12088} HPF is a cell-permeable aromatic amino-fluorescein derivative that has little intrinsic fluorescence.{11242} It undergoes oxidation only by highly reactive oxygen species (hROS) such as the hydroxyl radical, peroxynitrite, and hROS generated from a peroxidase/H2O2 system. It is inert to hypochlorite ion, nitric oxide, hydrogen peroxide (H2O2), superoxide, and other oxidants. Upon oxidation, HPF is converted to the highly fluorescent molecule fluorescein, with excitation/emission maxima of 490/515 nm, respectively, allowing the simple direct detection of highly reactive biological radicals.
Brand:CaymanSKU:10159 - 5 mgAvailable on backorder
The biology of highly reactive oxygen radical species is of great interest in many biomedical research disciplines, including neurodegeneration, aging, cancer, and infectious diseases.{9477} There are a number of fluorescent reagents, such as 2,7-dichlorodihydrofluorescein (DCDHF), that can be used to detect free radicals, but they have significant limitations due to their facile oxidation by light and numerous non-radical oxidants such as hydrogen peroxide (H2O2).{12088} HPF is a cell-permeable aromatic amino-fluorescein derivative that has little intrinsic fluorescence.{11242} It undergoes oxidation only by highly reactive oxygen species (hROS) such as the hydroxyl radical, peroxynitrite, and hROS generated from a peroxidase/H2O2 system. It is inert to hypochlorite ion, nitric oxide, hydrogen peroxide (H2O2), superoxide, and other oxidants. Upon oxidation, HPF is converted to the highly fluorescent molecule fluorescein, with excitation/emission maxima of 490/515 nm, respectively, allowing the simple direct detection of highly reactive biological radicals.
Brand:CaymanSKU:10159 - 500 µgAvailable on backorder
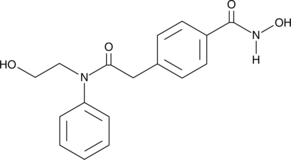
HPOB is a potent inhibitor of histone deacetylase 6 (HDAC6; IC50 = 56 nM).{28379} It is at least 30-fold less effective against other HDACs.{28379} Through its effects on HDAC6, HPOB induces acetylation of α-tubulin but not histones.{28379} HPOB reduces the growth, but not the viability, of normal and transformed cells.{28379} It enhances the death of transformed cells as triggered by the topoisomerase II inhibitors etoposide and doxorubicin.{28379} HPOB enhances the cytotoxicity of the broad spectrum HDAC inhibitor SAHA (Item No. 10009929) against cancer cells in nude mice carrying an androgen-dependent CWR22 human prostate cancer xenograft.{28379}
Brand:CaymanSKU:-HPOB is a potent inhibitor of histone deacetylase 6 (HDAC6; IC50 = 56 nM).{28379} It is at least 30-fold less effective against other HDACs.{28379} Through its effects on HDAC6, HPOB induces acetylation of α-tubulin but not histones.{28379} HPOB reduces the growth, but not the viability, of normal and transformed cells.{28379} It enhances the death of transformed cells as triggered by the topoisomerase II inhibitors etoposide and doxorubicin.{28379} HPOB enhances the cytotoxicity of the broad spectrum HDAC inhibitor SAHA (Item No. 10009929) against cancer cells in nude mice carrying an androgen-dependent CWR22 human prostate cancer xenograft.{28379}
Brand:CaymanSKU:-HPOB is a potent inhibitor of histone deacetylase 6 (HDAC6; IC50 = 56 nM).{28379} It is at least 30-fold less effective against other HDACs.{28379} Through its effects on HDAC6, HPOB induces acetylation of α-tubulin but not histones.{28379} HPOB reduces the growth, but not the viability, of normal and transformed cells.{28379} It enhances the death of transformed cells as triggered by the topoisomerase II inhibitors etoposide and doxorubicin.{28379} HPOB enhances the cytotoxicity of the broad spectrum HDAC inhibitor SAHA (Item No. 10009929) against cancer cells in nude mice carrying an androgen-dependent CWR22 human prostate cancer xenograft.{28379}
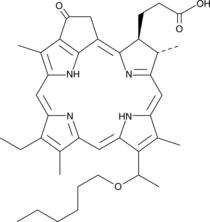
Brand:CaymanSKU:-HPPH is a chlorin that acts as a photosensitizer in photodynamic therapy (PDT) when stimulated with light at 655 nm.{39227} When administered systemically, HPPH accumulates in tumor cells and, when stimulated with light in the presence of oxygen, reactive oxygen species (ROS) are generated, leading to necrosis within the tumor. HPPH, at a dose of 0.5 mg/kg, increases survival in a nude rat model of glioma.{39228} It works synergistically with gemcitabine (Item No. 11690) in several pancreatic cancer cell lines to induce cell death.{39229}
Brand:CaymanSKU:20611 -Available on backorder
HPPH is a chlorin that acts as a photosensitizer in photodynamic therapy (PDT) when stimulated with light at 655 nm.{39227} When administered systemically, HPPH accumulates in tumor cells and, when stimulated with light in the presence of oxygen, reactive oxygen species (ROS) are generated, leading to necrosis within the tumor. HPPH, at a dose of 0.5 mg/kg, increases survival in a nude rat model of glioma.{39228} It works synergistically with gemcitabine (Item No. 11690) in several pancreatic cancer cell lines to induce cell death.{39229}
Brand:CaymanSKU:20611 -Available on backorder
HPPH is a chlorin that acts as a photosensitizer in photodynamic therapy (PDT) when stimulated with light at 655 nm.{39227} When administered systemically, HPPH accumulates in tumor cells and, when stimulated with light in the presence of oxygen, reactive oxygen species (ROS) are generated, leading to necrosis within the tumor. HPPH, at a dose of 0.5 mg/kg, increases survival in a nude rat model of glioma.{39228} It works synergistically with gemcitabine (Item No. 11690) in several pancreatic cancer cell lines to induce cell death.{39229}
Brand:CaymanSKU:20611 -Available on backorder
HPPH is a chlorin that acts as a photosensitizer in photodynamic therapy (PDT) when stimulated with light at 655 nm.{39227} When administered systemically, HPPH accumulates in tumor cells and, when stimulated with light in the presence of oxygen, reactive oxygen species (ROS) are generated, leading to necrosis within the tumor. HPPH, at a dose of 0.5 mg/kg, increases survival in a nude rat model of glioma.{39228} It works synergistically with gemcitabine (Item No. 11690) in several pancreatic cancer cell lines to induce cell death.{39229}
Brand:CaymanSKU:20611 -Available on backorder
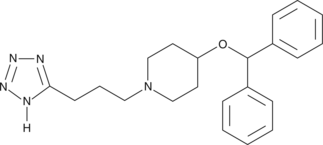
HQL-79 is a selective inhibitor of hematopoietic prostaglandin D (PGD) synthase. Structurally, it is a synthetic tetrazole compound originally prepared as a possible antihistamine.{11338} Subsequent evaluation in models of allergy and asthma demonstrates the HQL-79 is orally available, and inhibits the synthesis of PGD2 (Item No. 12010).{11337} HQL-79 is thus a likely lead compound for the preparation of potent, selective hematopoietic PGD synthase inhibitors.
Brand:CaymanSKU:10134 - 1 mgAvailable on backorder
HQL-79 is a selective inhibitor of hematopoietic prostaglandin D (PGD) synthase. Structurally, it is a synthetic tetrazole compound originally prepared as a possible antihistamine.{11338} Subsequent evaluation in models of allergy and asthma demonstrates the HQL-79 is orally available, and inhibits the synthesis of PGD2 (Item No. 12010).{11337} HQL-79 is thus a likely lead compound for the preparation of potent, selective hematopoietic PGD synthase inhibitors.
Brand:CaymanSKU:10134 - 10 mgAvailable on backorder
HQL-79 is a selective inhibitor of hematopoietic prostaglandin D (PGD) synthase. Structurally, it is a synthetic tetrazole compound originally prepared as a possible antihistamine.{11338} Subsequent evaluation in models of allergy and asthma demonstrates the HQL-79 is orally available, and inhibits the synthesis of PGD2 (Item No. 12010).{11337} HQL-79 is thus a likely lead compound for the preparation of potent, selective hematopoietic PGD synthase inhibitors.
Brand:CaymanSKU:10134 - 5 mgAvailable on backorder
HQL-79 is a selective inhibitor of hematopoietic prostaglandin D (PGD) synthase. Structurally, it is a synthetic tetrazole compound originally prepared as a possible antihistamine.{11338} Subsequent evaluation in models of allergy and asthma demonstrates the HQL-79 is orally available, and inhibits the synthesis of PGD2 (Item No. 12010).{11337} HQL-79 is thus a likely lead compound for the preparation of potent, selective hematopoietic PGD synthase inhibitors.
Brand:CaymanSKU:10134 - 50 mgAvailable on backorder
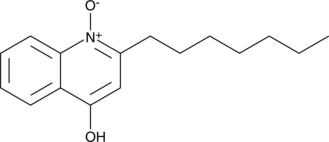
HQNO is an inhibitor of the respiratory chain binding to the mitochondrial cytochrome b protein, a component of complex III.{30589} In Vibrio alginolyticus, HQNO blocks 90% the activity of the sodium transporting NADH oxidase at a concentration of 40 μM.{24340} It is a useful tool for probing the mechanisms of electron transfer and proton or sodium translocation by the respiratory chain.
Brand:CaymanSKU:-HQNO is an inhibitor of the respiratory chain binding to the mitochondrial cytochrome b protein, a component of complex III.{30589} In Vibrio alginolyticus, HQNO blocks 90% the activity of the sodium transporting NADH oxidase at a concentration of 40 μM.{24340} It is a useful tool for probing the mechanisms of electron transfer and proton or sodium translocation by the respiratory chain.
Brand:CaymanSKU:-HQNO is an inhibitor of the respiratory chain binding to the mitochondrial cytochrome b protein, a component of complex III.{30589} In Vibrio alginolyticus, HQNO blocks 90% the activity of the sodium transporting NADH oxidase at a concentration of 40 μM.{24340} It is a useful tool for probing the mechanisms of electron transfer and proton or sodium translocation by the respiratory chain.
Brand:CaymanSKU:-Brand:CaymanSKU:660910 - 15 mlAvailable on backorder
HS-173 is an imidazopyridine analog that acts as a PI3Kα inhibitor with an IC50 value of 0.8 nM.{31012} It demonstrates antiproliferative activity in T47D, SK-BR-3, and MCF-7 cells with IC50 values of 0.6, 1.5, and 7.8 μM, respectively.{31012} HS-173 is reported to induce apoptosis by arresting the cell cycle at the G2/M phase and by activating caspases.{31013,31014} It has also been shown to block VEGF-induced angiogenesis both in vitro and in vivo.{31013}
Brand:CaymanSKU:-Available on backorder
HS-173 is an imidazopyridine analog that acts as a PI3Kα inhibitor with an IC50 value of 0.8 nM.{31012} It demonstrates antiproliferative activity in T47D, SK-BR-3, and MCF-7 cells with IC50 values of 0.6, 1.5, and 7.8 μM, respectively.{31012} HS-173 is reported to induce apoptosis by arresting the cell cycle at the G2/M phase and by activating caspases.{31013,31014} It has also been shown to block VEGF-induced angiogenesis both in vitro and in vivo.{31013}
Brand:CaymanSKU:-Available on backorder
HS-173 is an imidazopyridine analog that acts as a PI3Kα inhibitor with an IC50 value of 0.8 nM.{31012} It demonstrates antiproliferative activity in T47D, SK-BR-3, and MCF-7 cells with IC50 values of 0.6, 1.5, and 7.8 μM, respectively.{31012} HS-173 is reported to induce apoptosis by arresting the cell cycle at the G2/M phase and by activating caspases.{31013,31014} It has also been shown to block VEGF-induced angiogenesis both in vitro and in vivo.{31013}
Brand:CaymanSKU:-Available on backorder
HS-173 is an imidazopyridine analog that acts as a PI3Kα inhibitor with an IC50 value of 0.8 nM.{31012} It demonstrates antiproliferative activity in T47D, SK-BR-3, and MCF-7 cells with IC50 values of 0.6, 1.5, and 7.8 μM, respectively.{31012} HS-173 is reported to induce apoptosis by arresting the cell cycle at the G2/M phase and by activating caspases.{31013,31014} It has also been shown to block VEGF-induced angiogenesis both in vitro and in vivo.{31013}
Brand:CaymanSKU:-Available on backorder
Antigen: purified recombinant Hsf1 protein · Host: rat, clone 10H8 · Cross Reactivity: (+) human, mouse, rat, bovine, guinea pig, hamster, monkey, and rabbit Hsf1 · Applications: WB. IP, ICC, and EIA · Isotype: IgG1
Brand:CaymanSKU:10011433- 100 µgHeat shock factor 1 (Hsf1) belongs to a family of heat shock transcription factors that activate the transcription of genes encoding products required for protein folding, processing, targeting, degradation, and function.{15655} The up-regulation of Hsp expression by stressors is achieved at the level of transcription through a heat shock element (HSE) and a transcription factor.{15642,15643,15644} Most Hsfs have highly conserved amino acid sequences. On all Hsfs there is a DNA binding domain at the N-terminus. Hydrophobic repeats located adjacent to this binding domain are essential for the formation of active trimers. Towards the C-terminal region another short hydrophobic repeat exists and is thought to be necessary for suppression of trimerization.{15645} There are two main Hsfs, 1 and 2. Murine Hsf1 exists as two isoforms, however in higher eukaryotes Hsf1 is found in a diffuse cytoplasmic and nuclear distribution in unstressed cells. Once exposed to a multitude of stressors, it localizes to discrete nuclear granules within seconds. As it recovers from stress, Hsf1 dissipates from these granules to a diffuse nucleoplasmic distribution. Hsf2 on the other hand is similar to murine Hsf1, as it exists as two isoforms, the α form being more transcriptionally active than the smaller β form.{15656,15647} Various experiments have suggested that Hsf2 may have roles in differentiation and development.{15648,15649,15650}
Brand:CaymanSKU:10011433 - 100 µgAvailable on backorder
Antigen: purified recombinant Hsf1 protein · Host: rat, clone 10H8 · Cross Reactivity: (+) human, mouse, rat, bovine, guinea pig, hamster, monkey, and rabbit Hsf1 · Applications: WB. IP, ICC, and EIA · Isotype: IgG1
Brand:CaymanSKU:10011433- 100 µgAvailable on backorder
Antigen: purified recombinant Hsf1 protein · Host: rat, clone 10H8 · Cross Reactivity: (+) human, mouse, rat, bovine, guinea pig, hamster, monkey, and rabbit Hsf1 · Applications: WB. IP, ICC, and EIA · Isotype: IgG1
Brand:CaymanSKU:10011433- 25 µgHeat shock factor 1 (Hsf1) belongs to a family of heat shock transcription factors that activate the transcription of genes encoding products required for protein folding, processing, targeting, degradation, and function.{15655} The up-regulation of Hsp expression by stressors is achieved at the level of transcription through a heat shock element (HSE) and a transcription factor.{15642,15643,15644} Most Hsfs have highly conserved amino acid sequences. On all Hsfs there is a DNA binding domain at the N-terminus. Hydrophobic repeats located adjacent to this binding domain are essential for the formation of active trimers. Towards the C-terminal region another short hydrophobic repeat exists and is thought to be necessary for suppression of trimerization.{15645} There are two main Hsfs, 1 and 2. Murine Hsf1 exists as two isoforms, however in higher eukaryotes Hsf1 is found in a diffuse cytoplasmic and nuclear distribution in unstressed cells. Once exposed to a multitude of stressors, it localizes to discrete nuclear granules within seconds. As it recovers from stress, Hsf1 dissipates from these granules to a diffuse nucleoplasmic distribution. Hsf2 on the other hand is similar to murine Hsf1, as it exists as two isoforms, the α form being more transcriptionally active than the smaller β form.{15656,15647} Various experiments have suggested that Hsf2 may have roles in differentiation and development.{15648,15649,15650}
Brand:CaymanSKU:10011433 - 25 µgAvailable on backorder
Antigen: purified recombinant Hsf1 protein · Host: rat, clone 10H8 · Cross Reactivity: (+) human, mouse, rat, bovine, guinea pig, hamster, monkey, and rabbit Hsf1 · Applications: WB. IP, ICC, and EIA · Isotype: IgG1
Brand:CaymanSKU:10011433- 25 µgAvailable on backorder
Antigen: purified mouse recombinant Hsf2 · Host: rat, clone 3E2 · Cross Reactivity: (+) human, mouse, rat, guinea pig, hamster, monkey, rabbit, canine, bovine, ovine, and porcine Hsf2 · Application: WB · Isotype: IgG1
Brand:CaymanSKU:10011434- 100 µgHeat shock factor 2 (Hsf2) belongs to a family of heat shock transcription factors that activate the transcription of genes encoding products required for protein folding, processing, targeting, degradation, and function.{15655} The up-regulation of heat shock protein (Hsp) expression by stressors is achieved at the level of transcription through a heat shock element (HSE) and a transcription factor.{15642,15643,15644} Most Hsfs have highly conserved amino acid sequences. On all Hsfs there is a DNA binding domain at the N-terminus. Hydrophobic repeats located adjacent to this binding domain are essential for the formation of active trimers. Towards the C-terminal region another short hydrophobic repeat exists and is thought to be necessary for suppression of trimerization.{15645} There are two main Hsfs, 1 and 2. Murine Hsf1 exists as two isoforms, however in higher eukaryotes Hsf1 is found in a diffuse cytoplasmic and nuclear distribution in unstressed cells. Once exposed to a multitude of stressors, it localizes to discrete nuclear granules within seconds. As it recovers from stress, Hsf1 dissipates from these granules to a diffuse nucleoplasmic distribution. Hsf2 on the other hand is similar to murine Hsf1, as it exists as two isoforms, the α form being more transcriptionally active than the smaller β form.{15656,15647} Various experiments have suggested that Hsf2 may have roles in differentiation and development.{15648,15649,15650}
Brand:CaymanSKU:10011434 - 100 µgAvailable on backorder
Antigen: purified mouse recombinant Hsf2 · Host: rat, clone 3E2 · Cross Reactivity: (+) human, mouse, rat, guinea pig, hamster, monkey, rabbit, canine, bovine, ovine, and porcine Hsf2 · Application: WB · Isotype: IgG1
Brand:CaymanSKU:10011434- 100 µgAvailable on backorder
Antigen: purified mouse recombinant Hsf2 · Host: rat, clone 3E2 · Cross Reactivity: (+) human, mouse, rat, guinea pig, hamster, monkey, rabbit, canine, bovine, ovine, and porcine Hsf2 · Application: WB · Isotype: IgG1
Brand:CaymanSKU:10011434- 25 µgHeat shock factor 2 (Hsf2) belongs to a family of heat shock transcription factors that activate the transcription of genes encoding products required for protein folding, processing, targeting, degradation, and function.{15655} The up-regulation of heat shock protein (Hsp) expression by stressors is achieved at the level of transcription through a heat shock element (HSE) and a transcription factor.{15642,15643,15644} Most Hsfs have highly conserved amino acid sequences. On all Hsfs there is a DNA binding domain at the N-terminus. Hydrophobic repeats located adjacent to this binding domain are essential for the formation of active trimers. Towards the C-terminal region another short hydrophobic repeat exists and is thought to be necessary for suppression of trimerization.{15645} There are two main Hsfs, 1 and 2. Murine Hsf1 exists as two isoforms, however in higher eukaryotes Hsf1 is found in a diffuse cytoplasmic and nuclear distribution in unstressed cells. Once exposed to a multitude of stressors, it localizes to discrete nuclear granules within seconds. As it recovers from stress, Hsf1 dissipates from these granules to a diffuse nucleoplasmic distribution. Hsf2 on the other hand is similar to murine Hsf1, as it exists as two isoforms, the α form being more transcriptionally active than the smaller β form.{15656,15647} Various experiments have suggested that Hsf2 may have roles in differentiation and development.{15648,15649,15650}
Brand:CaymanSKU:10011434 - 25 µgAvailable on backorder
Antigen: purified mouse recombinant Hsf2 · Host: rat, clone 3E2 · Cross Reactivity: (+) human, mouse, rat, guinea pig, hamster, monkey, rabbit, canine, bovine, ovine, and porcine Hsf2 · Application: WB · Isotype: IgG1
Brand:CaymanSKU:10011434- 25 µgAvailable on backorder
The heat shock proteins (Hsps) act as molecular chaperones. Hsp90 is an abundant protein with roles in protein folding, cell signaling, and cancer. HSP-990 is an Hsp90 inhibitor with IC50 values of 0.6, 0.8, and 8.5 nM for Hsp90α, Hsp90β, and GRP94, respectively.{31009} It inhibits the TRAP1 ATPase with an IC50 value of 320 nM and demonstrates IC50 values of >5 µM in a panel of 51 unrelated kinases.{31009} In c-Met amplified GTL-16 gastric tumor cells, HSP-990 has been shown to dissociate the Hsp90-p23 complex, depleting the client protein c-Met and inducing Hsp70.{31009} HSP-990 can inhibit proliferation of various human tumor cell lines with GI50 values of 4-40 nM.{31009} Single oral administration of 15 mg/kg of HSP-990 was shown to induce sustained downregulation of c-Met and upregulation of Hsp70 in a GTL-16 xenograft model.{31009}
Brand:CaymanSKU:-Available on backorder
The heat shock proteins (Hsps) act as molecular chaperones. Hsp90 is an abundant protein with roles in protein folding, cell signaling, and cancer. HSP-990 is an Hsp90 inhibitor with IC50 values of 0.6, 0.8, and 8.5 nM for Hsp90α, Hsp90β, and GRP94, respectively.{31009} It inhibits the TRAP1 ATPase with an IC50 value of 320 nM and demonstrates IC50 values of >5 µM in a panel of 51 unrelated kinases.{31009} In c-Met amplified GTL-16 gastric tumor cells, HSP-990 has been shown to dissociate the Hsp90-p23 complex, depleting the client protein c-Met and inducing Hsp70.{31009} HSP-990 can inhibit proliferation of various human tumor cell lines with GI50 values of 4-40 nM.{31009} Single oral administration of 15 mg/kg of HSP-990 was shown to induce sustained downregulation of c-Met and upregulation of Hsp70 in a GTL-16 xenograft model.{31009}
Brand:CaymanSKU:-Available on backorder
The heat shock proteins (Hsps) act as molecular chaperones. Hsp90 is an abundant protein with roles in protein folding, cell signaling, and cancer. HSP-990 is an Hsp90 inhibitor with IC50 values of 0.6, 0.8, and 8.5 nM for Hsp90α, Hsp90β, and GRP94, respectively.{31009} It inhibits the TRAP1 ATPase with an IC50 value of 320 nM and demonstrates IC50 values of >5 µM in a panel of 51 unrelated kinases.{31009} In c-Met amplified GTL-16 gastric tumor cells, HSP-990 has been shown to dissociate the Hsp90-p23 complex, depleting the client protein c-Met and inducing Hsp70.{31009} HSP-990 can inhibit proliferation of various human tumor cell lines with GI50 values of 4-40 nM.{31009} Single oral administration of 15 mg/kg of HSP-990 was shown to induce sustained downregulation of c-Met and upregulation of Hsp70 in a GTL-16 xenograft model.{31009}
Brand:CaymanSKU:-Available on backorder
The heat shock proteins (Hsps) act as molecular chaperones. Hsp90 is an abundant protein with roles in protein folding, cell signaling, and cancer. HSP-990 is an Hsp90 inhibitor with IC50 values of 0.6, 0.8, and 8.5 nM for Hsp90α, Hsp90β, and GRP94, respectively.{31009} It inhibits the TRAP1 ATPase with an IC50 value of 320 nM and demonstrates IC50 values of >5 µM in a panel of 51 unrelated kinases.{31009} In c-Met amplified GTL-16 gastric tumor cells, HSP-990 has been shown to dissociate the Hsp90-p23 complex, depleting the client protein c-Met and inducing Hsp70.{31009} HSP-990 can inhibit proliferation of various human tumor cell lines with GI50 values of 4-40 nM.{31009} Single oral administration of 15 mg/kg of HSP-990 was shown to induce sustained downregulation of c-Met and upregulation of Hsp70 in a GTL-16 xenograft model.{31009}
Brand:CaymanSKU:-Available on backorder
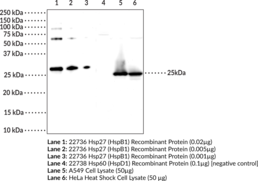
Heat shock protein 27 (Hsp27), also known as heat shock protein beta-1 (HspB1), is a member of the small heat shock protein (sHSP) family that is upregulated during conditions of cellular stress including heat shock, radiation, hypoxia, and exposure to reactive oxygen species (ROS).{40293,40294} It is composed of an N-terminal domain, a highly conserved alpha-crystallin domain, and a C-terminal domain. Hsp27 functions as a molecular chaperone to prevent protein aggregation in an ATPase-independent manner. This chaperone activity is altered by changes in oligomerization state or by post-translational modifications including phosphorylation at serine residues 15 and 82, which increases affinity for damaged polypeptides in response to heat shock.{40754} Hsp27 also works in complex with other chaperone proteins, such as Hsp70 (Item Nos. 22739 | 23002), to correct misfolded proteins. This protein also plays a role in apoptosis, proteasome activation, cell differentiation, and has been shown to interact with actin and intermediate filaments.{40295,40296,40297} Mutations in HSPB1 have been linked to hereditary neuromuscular diseases and cause Charcot-Marie-Tooth Disease Type 2 (CMT-2).{40298} Cayman’s Hsp27 (HspB1) Monoclonal Antibody can be used for Western blot and ELISA applications. The antibody recognizes Hsp27 (HspB1) at 25 kDa from human samples.
Brand:CaymanSKU:25692 - 100 µgAvailable on backorder
Immunogen: Full-length human recombinant Hsp27 protein • Host: Mouse • Species Reactivity: (+) Human • Cross Reactivity: (-) Hsp60 • Applications: ELISA, IF, IHC, and WB
Brand:CaymanSKU:25692- 100 µgAvailable on backorder
Immunogen: Full-length human recombinant Hsp27 protein • Host: Mouse • Species Reactivity: (+) Human • Cross Reactivity: (-) Hsp60 • Applications: ELISA, IF, IHC, and WB
Brand:CaymanSKU:25692- 100 µgHeat shock protein 27 (Hsp27), also known as heat shock protein beta-1 (HspB1), is a member of the small heat shock protein (sHSP) family that is upregulated during conditions of cellular stress including heat shock, radiation, hypoxia, and exposure to reactive oxygen species (ROS).{40293,40294} It is composed of an N-terminal domain, a highly conserved alpha-crystallin domain, and a C-terminal domain. Hsp27 functions as a molecular chaperone to prevent protein aggregation in an ATPase-independent manner. This chaperone activity is altered by changes in oligomerization state or by post-translational modifications including phosphorylation at serine residues 15 and 82, which increases affinity for damaged polypeptides in response to heat shock.{40754} Hsp27 also works in complex with other chaperone proteins, such as Hsp70 (Item Nos. 22739 | 23002), to correct misfolded proteins. This protein also plays a role in apoptosis, proteasome activation, cell differentiation, and has been shown to interact with actin and intermediate filaments.{40295,40296,40297} Mutations in HSPB1 have been linked to hereditary neuromuscular diseases and cause Charcot-Marie-Tooth Disease Type 2 (CMT-2).{40298} Cayman’s Hsp27 (HspB1) Polyclonal Antibody can be used for ELISA, Immunofluorescence, Immunohistochemistry, and Western blot applications. The antibody recognizes Hsp27 (HspB1) at 25 kDa from human samples.
Brand:CaymanSKU:24530 - 300 µgAvailable on backorder
Immunogen: Human recombinant Hsp27 (HspB1) • Host: Rabbit • Species reactivity: (+) Human • Applications: ELISA, IF, IHC and WB
Brand:CaymanSKU:24530- 300 µgAvailable on backorder
Immunogen: Human recombinant Hsp27 (HspB1) • Host: Rabbit • Species reactivity: (+) Human • Applications: ELISA, IF, IHC and WB
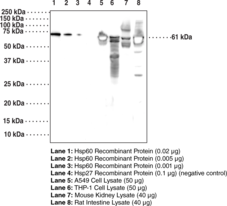
Brand:CaymanSKU:24530- 300 µgHeat shock protein 60 (Hsp60), also known as heat shock protein family D member 1 (HspD1), is an approximately 60 kDa protein that functions as a molecular chaperone.{35046} It belongs to the type I subclass of chaperonins and is found in eubacteria, mitochondria, and chloroplasts where its expression is induced by stress. Hsp60 primarily exists as a heptameric ring that it is converted to a tetradecameric double-ring structure in the presence of ATP.{36472} Within mitochondria, it associates with its co-chaperone, Hsp10, to form a barrel-like structure and refold proteins that have been shuttled to the mitochondria in an ATP-dependent manner.{35047,36472} Hsp60 also has extramitochondrial functions such as the production of proinflammatory cytokines in human leukocytes and activation of innate immune receptors.{35048,36473} Hsp60 expression is increased in the serum and saliva of patients with type 2 diabetes mellitus and mutations in HSPD1 lead to neurodegenerative diseases.{35049,36473} Cayman’s Hsp60 (HspD1) Monoclonal Antibody can be used for ELISA, IHC, and WB applications. The antibody recognizes Hsp60 (HspD1) at 61 kDa from human, mouse, and rat samples.
Brand:CaymanSKU:25693 - 100 µgAvailable on backorder
Immunogen: Full length human recombinant Hsp60 (HspD1) protein • Clone designation: 10C3 • Host: Mouse • Cross Reactivity: (-) Hsp27 • Species Reactivity: (+) Human, mouse, and rat Hsp60 (HspD1) • Isotype: IgG1 • Applications: ELISA, IHC, and WB
Brand:CaymanSKU:25693- 100 µgAvailable on backorder
Immunogen: Full length human recombinant Hsp60 (HspD1) protein • Clone designation: 10C3 • Host: Mouse • Cross Reactivity: (-) Hsp27 • Species Reactivity: (+) Human, mouse, and rat Hsp60 (HspD1) • Isotype: IgG1 • Applications: ELISA, IHC, and WB
Brand:CaymanSKU:25693- 100 µgHeat shock protein 60 (Hsp60), also known as heat shock protein family D member 1 (HspD1), is an approximately 60 kDa protein that functions as a molecular chaperone.{35046} It belongs to the type I subclass of chaperonins and is found in eubacteria, mitochondria, and chloroplasts where its expression is induced by stress. Hsp60 primarily exists as a heptameric ring that it is converted to a tetradecameric double-ring structure in the presence of ATP.{36472} Within mitochondria, it associates with its co-chaperone, Hsp10, to form a barrel-like structure and refold proteins that have been shuttled to the mitochondria in an ATP-dependent manner.{36472,35047} Hsp60 also has extramitochondrial functions such as the production of proinflammatory cytokines in human leukocytes and activation of innate immune receptors.{35048,36473} Hsp60 expression is increased in the serum and saliva of patients with type 2 diabetes mellitus and mutations in HSPD1 lead to neurodegenerative diseases.{36473,35049} Cayman’s Hsp60 (HspD1) Polyclonal Antibody can be used for Western blot and ELISA applications. The antibody recognizes Hsp60 (HspD1) at 60 kDa from human samples.
Brand:CaymanSKU:24531 - 100 µgAvailable on backorder
Immunogen: Human recombinant Hsp60 (HspD1) • Host: Rabbit • Species reactivity: (+) Human; other species not tested • Applications: ELISA, IHC, and WB,
Brand:CaymanSKU:24531- 100 µgAvailable on backorder
Immunogen: Human recombinant Hsp60 (HspD1) • Host: Rabbit • Species reactivity: (+) Human; other species not tested • Applications: ELISA, IHC, and WB,
Brand:CaymanSKU:24531- 100 µgHeat shock protein 70s (Hsp70s) are abundant and stress-inducible 70 kDa molecular chaperone proteins encoded by a highly conserved, multigene family.{15480} They are monomeric proteins that can be divided into two functional domains: an N-terminal ATPase domain and a substrate binding domain that contains a highly conserved EEVD motif at its C-terminus. Hsp70s are found in the cytosol, nuclei, endoplasmic reticulum, mitochondria, and chloroplasts of eukaryotes, as well as in bacteria. They function as molecular chaperones that assist in a wide range of cellular processes, including refolding of aggregated or misfolded proteins, co- and post-translational folding and assembly of nascent peptides, membrane translocation of secretory and organellar proteins, controlling activity of regulatory nuclear receptors, kinases and transcription factors, as well as cooperativity with the Hsp90 chaperone system in eukaryotes.{36132} The Hsp70 chaperone cycle is ATP-dependent and initiated by transient interaction of the Hsp70 substrate binding domain with hydrophobic regions within a peptide or protein. It consists of an alteration between the low-affinity ATP-bound state with fast rates of substrate exchange and the high-affinity ADP bound state with slow rates of substrate exchange. Hsp70s are subject to a variety of post-translational modifications and their expression is upregulated under conditions of cellular stress and in a variety of disease states. Cayman’s Hsp70 (HspA1A) Monoclonal Antibody (Clone 7F6) can be used for Western blot, ELISA, immunohistochemistry, and immunoprecipitation applications. The antibody recognizes Hsp70, also known as HspA1A, at ~70 kDa from human and mouse samples.
Brand:CaymanSKU:25694 - 100 µgAvailable on backorder
Immunogen: Full length human Hsp70 recombinant protein • Clone designation: 7F6 • Host: Mouse • Cross Reactivity: (-) GRP78 • Species reactivity: (+) Human, mouse Hsp70 (HspA1A); (-) Rat Hsp70 (HspA1A) • Isotype: IgG1 • Applications: ELISA, IP, IHC, and WB
Brand:CaymanSKU:25694- 100 µgAvailable on backorder
Immunogen: Full length human Hsp70 recombinant protein • Clone designation: 7F6 • Host: Mouse • Cross Reactivity: (-) GRP78 • Species reactivity: (+) Human, mouse Hsp70 (HspA1A); (-) Rat Hsp70 (HspA1A) • Isotype: IgG1 • Applications: ELISA, IP, IHC, and WB
Brand:CaymanSKU:25694- 100 µgHeat shock protein 70s (Hsp70s) are abundant and stress-inducible 70 kDa molecular chaperone proteins encoded by a highly conserved, multigene family.{15480} They are monomeric proteins that can be divided into two functional domains: an N-terminal ATPase domain and a substrate binding domain that contains a highly conserved EEVD motif at its C-terminus. Hsp70s are found in the cytosol, nuclei, endoplasmic reticulum, mitochondria, and chloroplasts of eukaryotes, as well as in bacteria. They function as molecular chaperones that assist in a wide range of cellular processes, including refolding of aggregated or misfolded proteins, co- and post-translational folding and assembly of nascent peptides, membrane translocation of secretory and organellar proteins, controlling activity of regulatory nuclear receptors, kinases and transcription factors, as well as cooperativity with the Hsp90 chaperone system in eukaryotes.{36132} The Hsp70 chaperone cycle is ATP-dependent and initiated by transient interaction of the Hsp70 substrate binding domain with hydrophobic regions within a peptide or protein. It consists of an alteration between the low-affinity ATP-bound state with fast rates of substrate exchange and the high-affinity ADP bound state with slow rates of substrate exchange. Hsp70s are subject to a variety of post-translational modifications and their expression is upregulated under conditions of cellular stress and in a variety of disease states. Cayman’s Hsp70 (HspA1A) Polyclonal Antibody can be used for ELISA, Immunofluorescence, Immunohistochemistry, and Western blot applications. The antibody recognizes Hsp70, also known as HspA1A, at ~70 kDa from human samples.
Brand:CaymanSKU:24532 - 200 µgAvailable on backorder
Immunogen: Human recombinant protein • Host: Rabbit • Species Reactivity: (+) Human and rat • Applications: ELISA, IF, IHC, and WB
Brand:CaymanSKU:24532- 200 µgAvailable on backorder
Immunogen: Human recombinant protein • Host: Rabbit • Species Reactivity: (+) Human and rat • Applications: ELISA, IF, IHC, and WB
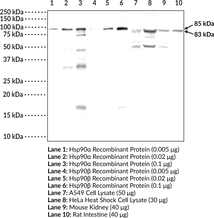
Brand:CaymanSKU:24532- 200 µgHsp90 is a multidomain protein that functions as a molecular chaperone to assist in folding and activation of nascent peptides, refolding unfolded or misfolded proteins, and preventing protein aggregation.{15502} Hsp90α is the inducible cytosolic isoform of Hsp90 while Hsp90β is the constitutively active cytosolic isoform. Hsp90α and β are encoded by HSP90AA and HSP90AB, respectively in humans.{17930} C-terminal dimerization of Hsp90, coupled with ATPase molecular clamp activity induces a conformational change in the N-terminal nucleotide binding domain that facilitates substrate binding and initiates the chaperone cycle.{17932} Hsp90 interacts with many co-chaperones during its chaperone cycle including p23 and Sba1, which help recruit substrates to the Hsp90 complex, Hsp70 (Item Nos. 22739 | 23002), which loads nascent polypeptides onto the Hsp90 dimer, and the ATPase activator Aha1 that promotes ATP hydrolysis and substrate release.{17931,41851} Hsp90 is overexpressed in cancer cells and stabilizes client proteins that promote oncogenesis, including transcription factors, signaling proteins, and kinases.{17930,41851} Hsp90 also decreases α-synuclein fibril formation and toxicity as well as Q35 aggregation in in vitro models of Parkinson’s and Huntington’s disease, respectively, implying a role in neurodegenerative disease.{41852} Cayman’s Hsp90α/β Polyclonal Antibody can be used for Western blot and ELISA applications. This antibody recognizes Hsp90α at 85 kDa and Hsp90β at 83 kDa from human, mouse, and rat samples.
Brand:CaymanSKU:24559 - 500 µlAvailable on backorder
Immunogen: Recombinant full-length human Hsp90α and Hsp90β proteins • Host: Rabbit • Species Reactivity: (+) Human, mouse, and rat • Cross Reactivity: (+) Hsp90α and Hsp90β • Applications: ELISA, IF, IHC, and WB
Brand:CaymanSKU:24559- 500 µlAvailable on backorder
Immunogen: Recombinant full-length human Hsp90α and Hsp90β proteins • Host: Rabbit • Species Reactivity: (+) Human, mouse, and rat • Cross Reactivity: (+) Hsp90α and Hsp90β • Applications: ELISA, IF, IHC, and WB
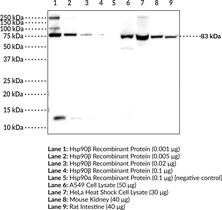
Brand:CaymanSKU:24559- 500 µlHeat shock protein 90 α (Hsp90α) is the inducible cytosolic isoform of Hsp90 that is encoded by HSP90AA in humans.{17930} Hsp90 is a multidomain protein that functions as a molecular chaperone to assist in folding and activation of nascent peptides, refolding unfolded or misfolded proteins, and preventing protein aggregation.{15502} C-terminal dimerization of Hsp90, coupled with ATPase molecular clamp activity induces a conformational change in the N-terminal nucleotide binding domain that facilitates substrate binding and initiates the chaperone cycle.{17932} Hsp90 interacts with many co-chaperones during its chaperone cycle including p23 and Sba1, which help recruit substrates to the Hsp90 complex, Hsp70 (Item Nos. 22739 | 23002), which loads nascent polypeptides onto the Hsp90 dimer, and the ATPase activator Aha1 that promotes ATP hydrolysis and substrate release.{17931,41851} Hsp90 is overexpressed in cancer cells and stabilizes client proteins that promote oncogenesis, including transcription factors, signaling proteins, and kinases.{17930,41851} Hsp90 also decreases α-synuclein fibril formation and toxicity as well as Q35 aggregation in in vitro models of Parkinson’s and Huntington’s disease, respectively, implying a role in neurodegenerative disease.{41852} Cayman’s Hsp90α Polyclonal Antibody can be used for Western blot and ELISA applications. This antibody recognizes Hsp90α at 85 kDa from human, mouse, and rat samples.
Brand:CaymanSKU:25725 - 500 µlAvailable on backorder
Immunogen: Human recombinant Hsp90α protein • Host: Rabbit • Species reactivity: (+) Human, mouse, rat • Cross reactivity: (-) Hsp90β • Applications: ELISA, IF, IHC, and WB,
Brand:CaymanSKU:25725- 500 µlAvailable on backorder
Immunogen: Human recombinant Hsp90α protein • Host: Rabbit • Species reactivity: (+) Human, mouse, rat • Cross reactivity: (-) Hsp90β • Applications: ELISA, IF, IHC, and WB,
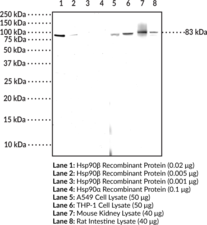
Brand:CaymanSKU:25725- 500 µlHeat shock protein 90 β (Hsp90β) is the constitutively active cytosolic isoform of Hsp90 that is encoded by HSP90AB in humans.{17930} Hsp90 is a multidomain protein that functions as a molecular chaperone to assist in folding and activation of nascent peptides, refolding unfolded or misfolded proteins, and preventing protein aggregation.{15502} C-terminal dimerization of Hsp90, coupled with ATPase molecular clamp activity induces a conformational change in the N-terminal nucleotide binding domain that facilitates substrate binding and initiates the chaperone cycle.{17932} Hsp90 interacts with many co-chaperones during its chaperone cycle including p23 and Sba1, which help recruit substrates to the Hsp90 complex, Hsp70 (Item Nos. 22739 | 23002), which loads nascent polypeptides onto the Hsp90 dimer, and the ATPase activator Aha1 that promotes ATP hydrolysis and substrate release.{17931,41851} Hsp90 is overexpressed in cancer cells and stabilizes client proteins that promote oncogenesis, including transcription factors, signaling proteins, and kinases.{17930,41851} Hsp90 also decreases α-synuclein fibril formation and toxicity as well as Q35 aggregation in in vitro models of Parkinson’s and Huntington’s disease, respectively, implying a role in neurodegenerative disease.{41852} Cayman’s Hsp90β Monoclonal Antibody (Clone8D6) can be used for Western blot and ELISA applications. This antibody recognizes Hsp90β at 83 kDa from human, mouse, and rat samples.
Brand:CaymanSKU:25695 - 100 µgAvailable on backorder
Immunogen: Full-length human recombinant Hsp90β protein • Host: Mouse • Species reactivity: (+) Human, mouse, rat • Cross reactivity: (-) Hsp90α • Applications: ELISA and WB,
Brand:CaymanSKU:25695- 100 µgAvailable on backorder
Immunogen: Full-length human recombinant Hsp90β protein • Host: Mouse • Species reactivity: (+) Human, mouse, rat • Cross reactivity: (-) Hsp90α • Applications: ELISA and WB,
Brand:CaymanSKU:25695- 100 µgHeat shock protein 90 β (Hsp90β) is the constitutively active cytosolic isoform of Hsp90 that is encoded by HSP90AB in humans.{17930} Hsp90 is a multidomain protein that functions as a molecular chaperone to assist in folding and activation of nascent peptides, refolding unfolded or misfolded proteins, and preventing protein aggregation.{15502} C-terminal dimerization of Hsp90, coupled with ATPase molecular clamp activity induces a conformational change in the N-terminal nucleotide binding domain that facilitates substrate binding and initiates the chaperone cycle.{17932} Hsp90 interacts with many co-chaperones during its chaperone cycle including p23 and Sba1, which help recruit substrates to the Hsp90 complex, Hsp70 (Item Nos. 22739 | 23002), which loads nascent polypeptides onto the Hsp90 dimer, and the ATPase activator Aha1 that promotes ATP hydrolysis and substrate release.{17931,41851} Hsp90 is overexpressed in cancer cells and stabilizes client proteins that promote oncogenesis, including transcription factors, signaling proteins, and kinases.{17930,41851} Hsp90 also decreases α-synuclein fibril formation and toxicity as well as Q35 aggregation in in vitro models of Parkinson’s and Huntington’s disease, respectively, implying a role in neurodegenerative disease.{41852} Cayman’s Hsp90β Polyclonal Antibody can be used for Western blot, ELISA, IHC, and IF applications. This antibody recognizes Hsp90β at 83 kDa from human, mouse, and rat samples.
Brand:CaymanSKU:25730 - 500 µLAvailable on backorder
Immunogen: Recombinant full-length human Hsp90β protein • Host: Rabbit • Species reactivity: (+) Human, mouse, and rat • Cross reactivity: (-) Hsp90α • Applications: ELISA, IHC, IF, and WB
Brand:CaymanSKU:25730- 500 µLAvailable on backorder
Immunogen: Recombinant full-length human Hsp90β protein • Host: Rabbit • Species reactivity: (+) Human, mouse, and rat • Cross reactivity: (-) Hsp90α • Applications: ELISA, IHC, IF, and WB
Brand:CaymanSKU:25730- 500 µLHT-2 toxin is a type A trichothecene mycotoxin and an active, deacetylated metabolite of the trichothecene mycotoxin T-2 toxin (Item No. 11444).{53282,31995} Like T-2 toxin, HT-2 toxin inhibits protein synthesis and cell proliferation in plants.{31995} HT-2 toxin also reduces viability of HepG2, A549, HEp-2, Caco-2, A-204, U937, Jurkat, and RPMI-8226 cancer cells with IC50 values ranging from 3.1 to 23 ng/ml and human umbilical vein endothelial cells (HUVECs) with an IC50 value of 56.4 ng/ml.{53282} It induces oxidative stress, DNA damage, and autophagy in, as well as halts the development of, cultured mouse embryos when used at a concentration of 10 nM.{59456} HT-2 toxin has been found in cereal grains and food products.{59457,59458}
Brand:CaymanSKU:20431 -Available on backorder
HT-2 toxin is a type A trichothecene mycotoxin and an active, deacetylated metabolite of the trichothecene mycotoxin T-2 toxin (Item No. 11444).{53282,31995} Like T-2 toxin, HT-2 toxin inhibits protein synthesis and cell proliferation in plants.{31995} HT-2 toxin also reduces viability of HepG2, A549, HEp-2, Caco-2, A-204, U937, Jurkat, and RPMI-8226 cancer cells with IC50 values ranging from 3.1 to 23 ng/ml and human umbilical vein endothelial cells (HUVECs) with an IC50 value of 56.4 ng/ml.{53282} It induces oxidative stress, DNA damage, and autophagy in, as well as halts the development of, cultured mouse embryos when used at a concentration of 10 nM.{59456} HT-2 toxin has been found in cereal grains and food products.{59457,59458}
Brand:CaymanSKU:20431 -Available on backorder
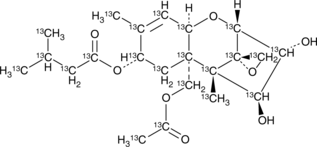
HT-2 toxin-13C22 is intended for use as an internal standard for the quantification of HT-2 toxin (Item No. 20431) by GC- or LC-MS. HT-2 toxin is a type A trichothecene mycotoxin and an active, deacetylated metabolite of the trichothecene mycotoxin T-2 toxin (Item No. 11444).{53282,31995} Like T-2 toxin, HT-2 toxin inhibits protein synthesis and cell proliferation in plants.{31995} HT-2 toxin also reduces viability of HepG2, A549, HEp-2, Caco-2, A-204, U937, Jurkat, and RPMI-8226 cancer cells with IC50 values ranging from 3.1 to 23 ng/ml and human umbilical vein endothelial cells with an IC50 value of 56.4 ng/ml.{53282} It induces oxidative stress, DNA damage, and autophagy in, as well as halts the development of, cultured mouse embryos when used at a concentration of 10 nM.{59456} HT-2 toxin has been found in cereal grains and food products.{59457,59458}
Brand:CaymanSKU:31299 - 1.2 mlAvailable on backorder
NUAK1 (also known as AMPK-related kinase 5) and NUAK2 (also known as SNF1/AMPK-related kinase) are members of the AMP-activated protein kinase (AMPK) family of protein kinases that are activated by the liver kinase B1 tumor suppressor kinase. NUAK kinases are thought to have roles in regulating cell adhesion, cancer cell invasion, embryonic development, senescence, proliferation, neuronal polarity, and axon branching. HTH-01-015 is a selective inhibitor of NUAK1 (IC50 = 100 nM) and does not affect the activity of a panel of 139 other kinases, including additional AMPK family members.{30771} At 10 µM, HTH-01-015 has been shown to partially inhibit the phosphorylation of the NUAK1 substrate, myosin phosphate-targeting subunit 1 at Ser445.{30771} When administered to mouse embryonic fibroblasts in vitro, 10 µM HTH-01-015 inhibits proliferation and migration in a wound-healing assay.{30771} It has also been shown to impair the invasive potential of U2OS cells at similar concentrations in a 3D cell invasion assay.{30771}
Brand:CaymanSKU:-Available on backorder
NUAK1 (also known as AMPK-related kinase 5) and NUAK2 (also known as SNF1/AMPK-related kinase) are members of the AMP-activated protein kinase (AMPK) family of protein kinases that are activated by the liver kinase B1 tumor suppressor kinase. NUAK kinases are thought to have roles in regulating cell adhesion, cancer cell invasion, embryonic development, senescence, proliferation, neuronal polarity, and axon branching. HTH-01-015 is a selective inhibitor of NUAK1 (IC50 = 100 nM) and does not affect the activity of a panel of 139 other kinases, including additional AMPK family members.{30771} At 10 µM, HTH-01-015 has been shown to partially inhibit the phosphorylation of the NUAK1 substrate, myosin phosphate-targeting subunit 1 at Ser445.{30771} When administered to mouse embryonic fibroblasts in vitro, 10 µM HTH-01-015 inhibits proliferation and migration in a wound-healing assay.{30771} It has also been shown to impair the invasive potential of U2OS cells at similar concentrations in a 3D cell invasion assay.{30771}
Brand:CaymanSKU:-Available on backorder
NUAK1 (also known as AMPK-related kinase 5) and NUAK2 (also known as SNF1/AMPK-related kinase) are members of the AMP-activated protein kinase (AMPK) family of protein kinases that are activated by the liver kinase B1 tumor suppressor kinase. NUAK kinases are thought to have roles in regulating cell adhesion, cancer cell invasion, embryonic development, senescence, proliferation, neuronal polarity, and axon branching. HTH-01-015 is a selective inhibitor of NUAK1 (IC50 = 100 nM) and does not affect the activity of a panel of 139 other kinases, including additional AMPK family members.{30771} At 10 µM, HTH-01-015 has been shown to partially inhibit the phosphorylation of the NUAK1 substrate, myosin phosphate-targeting subunit 1 at Ser445.{30771} When administered to mouse embryonic fibroblasts in vitro, 10 µM HTH-01-015 inhibits proliferation and migration in a wound-healing assay.{30771} It has also been shown to impair the invasive potential of U2OS cells at similar concentrations in a 3D cell invasion assay.{30771}
Brand:CaymanSKU:-Available on backorder
HTMT is a histamine H1 receptor agonist.{47692} It also binds to the histamine H4 receptor (Ki = 1.2 µM) but does not increase calcium mobilization in HEK293 cells expressing the receptor at 10 µM.{47693} HTMT increases intracellular calcium and inositol phosphate levels (EC50s = 19 and 30 µM, respectively) in human peripheral blood lymphocytes.{47694} It increases proliferation of immortalized mouse small, but not large, cholangiocytes, when used at a concentration of 10 µM.{47696} HTMT (10 µg/kg twice per day) increases levels of sheep red blood cell-induced IgG and IgM antibodies in rabbit serum.{47697} It induces scratching in mice when administered at an intradermal dose of 0.2 µmol, an effect that is reduced by the H1 receptor antagonist terfenadine (Item No. 20305).{47695}
Brand:CaymanSKU:29018 - 1 mgAvailable on backorder
HTMT is a histamine H1 receptor agonist.{47692} It also binds to the histamine H4 receptor (Ki = 1.2 µM) but does not increase calcium mobilization in HEK293 cells expressing the receptor at 10 µM.{47693} HTMT increases intracellular calcium and inositol phosphate levels (EC50s = 19 and 30 µM, respectively) in human peripheral blood lymphocytes.{47694} It increases proliferation of immortalized mouse small, but not large, cholangiocytes, when used at a concentration of 10 µM.{47696} HTMT (10 µg/kg twice per day) increases levels of sheep red blood cell-induced IgG and IgM antibodies in rabbit serum.{47697} It induces scratching in mice when administered at an intradermal dose of 0.2 µmol, an effect that is reduced by the H1 receptor antagonist terfenadine (Item No. 20305).{47695}
Brand:CaymanSKU:29018 - 10 mgAvailable on backorder