Description
TMPD is an easily oxidizable compound that serves as a reducing co-substrate for heme peroxidases.{8584} TMPD undergoes one-electron oxidation by the heme peroxidase higher oxidation states (compounds I and II) producing a highly colored product that absorbs at 611 nm.{8581} Thus, the stoichiometry of oxidation is 2 moles of TMPD oxidized per mole of hydroperoxide reduced by the peroxidase. The extinction coefficient of the oxidized TMPD at 611 nm is 12,200.{8581} TMPD is also used for the detection of peroxidases on polyacrylamide gels.{8585}
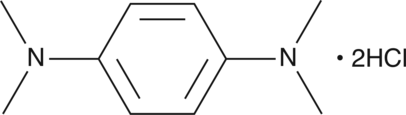
Formal name: N,N,N’,N’-tetramethyl-1,4-benzenediamine, dihydrochloride
Synonyms: N,N,N’,N’-Tetramethyl-p-Phenylenediamine|Wurster’s Reagent
Molecular weight: 237.2
CAS: 637-01-4
Purity: ≥95%
Formulation: A crystalline solid