Description
TMB is an aromatic amine that undergoes oxidation by the higher oxidation states of heme peroxidases (compounds I and II) thereby serving as a reducing co-substrate. One electron oxidation of TMB results in a radical cation that forms a charge-transfer complex with the unoxidized compound. This charge transfer complex absorbs at 652 nm (ε = 39,000).{8111} The completely oxidized form (diimine) absorbs at 450 nm (ε = 59,000) and is formed by two sequential one-electron oxidations of TMB.{8111,8109} Thus the stoichiometry of oxidation is 0.5 mole charge transfer complex (λmax = 652 nm) or 1 mole of diimine (λmax = 450 nm) formed (or TMB oxidized) per mole of hydroperoxide reduced by the peroxidase.
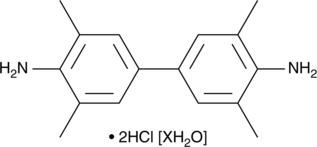
Formal name: 3,3′,5,5′-tetramethyl-[1,1′-biphenyl]-4,4′-diamine, dihydrochloride, dihydrate
Synonyms:
Molecular weight: 349.3
CAS: 207738-08-7
Purity: ≥98%
Formulation: A crystalline solid