Description
Peroxidation of common ω-6 PUFAs such as linoleic acid, DGLA, and arachidonic acid can give rise to 4-HNE. 4-HNE is cleared rapidly from the plasma and undergoes enterohepatic circulation as a glutathione conjugate in the rat.{7334} About two thirds of an administered dose of 4-HNE is excreted within 48 hours in the urine, primarily in the form of mercapturic acid conjugates.{8626} The C-1 aldehyde of 4-HNE is reduced to an alcohol in about half of these metabolites. The remainder are C-1 aldehydes or have been oxidized to C-1 carboxylic acids. These aldehydes and carboxylic acids can also form γ-lactols and γ-lactones, respectively, producing at least 4 or 5 end urinary metabolites of 4-HNE in vivo.
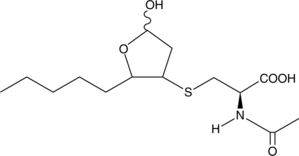
Formal name: N-acetyl-S-(tetrahydro-5-hydroxy-2-pentyl-3-furanyl)-L-cysteine
Synonyms:
Molecular weight: 319.4
CAS: 146764-24-1
Purity: ≥98%
Formulation: A solution in ethanol