Description
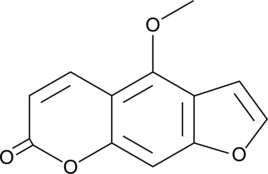
A furanocoumarin derivative with diverse biological activities; decreases the viability of DLD-1 and LoVo colorectal cancer cells in a concentration-dependent manner, halts the cell cycle at the G0/G1 and sub-G1 phases, and induces apoptosis at 50 μM; has photosensitizing activity; increases the number of sunburn cells in guinea pig skin in response to UVA radiation; increases the pain threshold in assays for mechanical, cold, and hot allodynia, as well as mechanical hyperalgesia, in a rat model of vincristine-induced neuropathic pain at 10 mg/kg; inhibits vincristine-induced increases in plasma TNF-α and IL-1β levels and decreases in GSH levels in the spinal cord and sciatic nerve in rats; inhibits CYP3A4 (IC50 = ~25 μM) in human liver microsomes
Formal name: 4-methoxy-7H-furo[3,2-g][1]benzopyran-7-one
Synonyms: 5-Methoxypsoralen|5-MOP|Heraclin|NSC 95437
Molecular weight: 216.2
CAS: 484-20-8
Purity: ≥98%
Formulation: A crystalline solid
Product Type|Biochemicals|Natural Products|Coumarins||Product Type|Biochemicals|Small Molecule Inhibitors|Cytochrome P450||Research Area|Cancer|Cell Cycle|G1||Research Area|Cancer|Cell Death|Apoptosis||Research Area|Immunology & Inflammation||Research Area|Neuroscience|Pain Research||Research Area|Oxidative Stress & Reactive Species|Reactive Sulfur|Glutathione||Research Area|Toxicology|Drug Metabolism|Cytochrome P450