Description
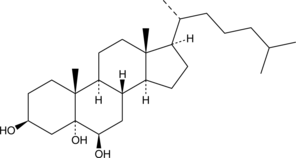
An oxysterol metabolite of cholesterol; inhibits NMDA-mediated calcium influx in HEK293 cells expressing NR1/NR2B NMDA receptors; binds to Nav channels and decreases action potentials in hippocampal neurons in vitro at 10 µM; increases survival of spinal cord motoneurons, cortical neurons, and cerebellar granule neurons in vitro at 5-15 µM; neuroprotective in a rat model of cerebral ischemia at 12 mg/kg ; increases latency to seizure onset and reduces severity of PTZ-induced seizures in rats; has been used as a replacement for cholesterol in the study of cholesterol binding proteins
Formal name: cholestane-3β,5α,6β-triol
Synonyms: 5α,6β-di-OHC|Cholestanetriol|NSC 124751|NSC 18178
Molecular weight: 420.7
CAS: 1253-84-5
Purity: ≥95%
Formulation: A crystalline solid
Product Type|Biochemicals|Ion Channel Modulation|Blockers||Product Type|Biochemicals|Lipids|Sterol Lipids||Research Area|Lipid Biochemistry|Sterol Lipids||Research Area|Neuroscience|Neuroprotection|Ischemia||Research Area|Neuroscience|Seizure Disorders