Description
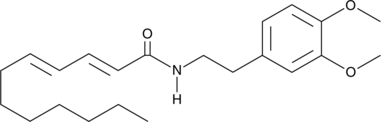
A potent endocannabinoid uptake inhibitor (IC50s = 10 and 283 nM for AEA and 2-AG, respectively, in U937 cells); inhibits AEA uptake in FAAH-deficient HMC-1 human mast cells and Neuro2a mouse neuroblastoma cells (IC50s = 137 and 55 nM, respectively); increases AEA and 2-AG levels by 1.5-fold in mouse brain but not peripheral tissues (10 mg/kg, i.p. for seven days); induces a typical tetrad of hypothermia, catalepsy, analgesia, and hypomotility in mice
Formal name: (2E,4E)-N-[2-(3,4-dimethoxyphenyl)ethyl]-2,4-dodecadienamide
Synonyms:
Molecular weight: 359.5
CAS: 2108100-73-6
Purity: ≥97%
Formulation: A crystalline solid
Product Type|Biochemicals|Receptor Pharmacology|Agonists||Product Type|Biochemicals|Transporter & Exchanger Modulators||Research Area|Neuroscience|Cannabinoid Research|CB1 & CB2 Receptors||Research Area|Neuroscience|Cannabinoid Research|Endocannabinoids||Research Area|Neuroscience|Pain Research