Description
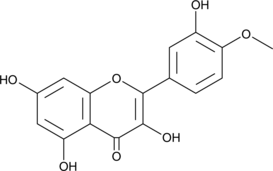
A flavonoid from C. ordata with anticancer and antiplasmodial activity; inhibits the viability of HL-60, U937, MOLT-3, Raji, K562, MCF-7, SK-MEL-1, and A549 human tumor cell lines with IC50 values ranging from 5.5-24.1 μM; induces G2-M arrest and inhibits tubulin polymerization in vitro; inhibits breast cancer resistance protein (BCRP/ABCG2; IC50 = 0.040 μM in a vesicular transport assay); reduces in vitro proliferation of chloroquine-resistant P. falciparum (IC50 = 4.8 μM),
Formal name: 3,5,7-trihydroxy-2-(3-hydroxy-4-methoxyphenyl)-4H-1-benzopyran-4-one
Synonyms: 4′-methoxy Quercetin|Tamarixetin
Molecular weight: 316.3
CAS: 603-61-2
Purity: ≥98%
Formulation: A solid
Product Type|Biochemicals|Antiparasitics|Antiprotozoals||Product Type|Biochemicals|Natural Products|Flavonoids||Product Type|Biochemicals|Transporter & Exchanger Modulators||Research Area|Cancer|Cell Cycle|G2/M||Research Area|Immunology & Inflammation||Research Area|Infectious Disease|Parasitic Diseases|Malaria||Research Area|Toxicology|Drug Metabolism|Drug Metabolites