Description
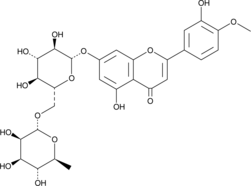
A flavonoid with diverse biological activities; inhibits LPS-induced production of PGE2, NO, IL-12, IL-6, and TNF-α in RAW 264.7 macrophages from 10-50 µM; reduces gastric ulcer area and leukocyte invasion in a rat model of ethanol-induced gastric injury at 100 mg/kg; decreases lung MDA levels, increases lung GSH levels and catalase activity, and reduces fibrosis in a mouse model of paraquat-induced lung injury; increases the time spent in the goal quarter in a spatial memory test and reverses hippocampal dentate gyrus long term potentiation impairments in a rat model of scopolamine-induced cognitive impairment at 50 and 100 mg/kg; reduces mechanical and thermal hyperalgesia in a rat model of neuropathic pain induced by chronic constriction injury
Formal name: 7-[[6-O-(6-deoxy-α-L-mannopyranosyl)-β-D-glucopyranosyl]oxy]-5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-4H-1-benzopyran-4-one
Synonyms: 3’,5,7-Trihydroxy-4’-methoxyflavone 7-rutinoside
Molecular weight: 608.5
CAS: 520-27-4
Purity: ≥90%
Formulation: A crystalline solid
Product Type|Biochemicals|Natural Products|Flavonoids||Product Type|Biochemicals|Ox Stress Reagents|Antioxidants||Research Area|Cell Biology|Cell Signaling|Nitric Oxide Signaling||Research Area|Immunology & Inflammation|Gastric Disease|Peptic Ulcers||Research Area|Immunology & Inflammation|Inflammatory Lipid Mediators|Prostaglandins||Research Area|Immunology & Inflammation|Innate Immunity||Research Area|Immunology & Inflammation|Pulmonary Diseases||Research Area|Neuroscience|Behavioral Neuroscience|Learning & Memory||Research Area|Neuroscience|Pain Research||Research Area|Oxidative Stress & Reactive Species|Antioxidant Activity||Research Area|Oxidative Stress & Reactive Species|Lipid Peroxidation||Research Area|Oxidative Stress & Reactive Species|Reactive Oxygen|Catalase||Research Area|Oxidative Stress & Reactive Species|Reactive Sulfur|Glutathione