Description
An inhibitor of neutral sphingomyelinase (IC50 = 1 μM); selective for neutral sphingomyelinase over acid sphingomyelinase at concentrations up to 150 μM as well as B. cereus PC-PLC, human lyso-PAF PLC, and bovine PP2A at 10 μM; inhibits TNF-α-induced sphingomyelin hydrolysis and TNF-α-induced cell death in MCF-7 cells; reduces the inhibitory effects of OxPAPC and KOdiA-PE on LPS-induction of IL-8 in human aortic endothelial cells; reverses hypoxia-induced pulmonary vasoconstriction in rats at 1 mg/kg,
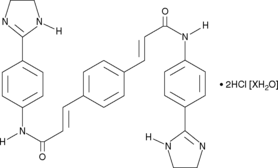
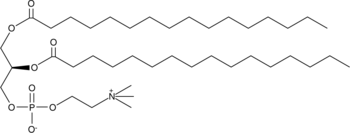
Formal name: 3,3′-(1,4-phenylene)bis[N-[4-(4,5-dihydro-1H-imidazol-2-yl)phenyl]-dihydrochloride-2-propenamide
Synonyms:
Molecular weight: 577.5
CAS: 6823-69-4
Purity: ≥90%
Formulation: A crystalline solid
Product Type|Biochemicals|Small Molecule Inhibitors|Sphingolipid Turnover||Research Area|Immunology & Inflammation