Description
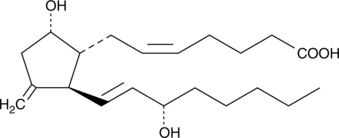
Prostaglandin D2 (PGD2) is one of the five primary enzymatic prostaglandins derived directly from PGH2. PGD2 is produced abundantly in the CSF by the lipocalin-type PGD synthase, and in the periphery by myeloid cells including mast cells and basophils by a second, leukocyte-type PGD synthase.{9588} PGD2 is chemically unstable, and its use and analysis is complicated by its short in vivo half-life. 11-deoxy-11-methylene PGD2 is a novel, chemically stable, isosteric analog of PGD2 wherein the 11-keto group is replaced by an exocyclic methylene. In the PGE series, the analogous modification leads to a stable, somewhat less potent agonist which embodies the same uterine stimulant and cervical ripening activities as the parent prostaglandin.{7894} However, 11-deoxy-11-methylene PGD2 has been reported by one group to be essentially without agonist activity on human platelets, a DP1 receptor assay.{11175} The CRTH2-receptor actions of 11-deoxy-11-methylene PGD2 are not yet reported.
Formal name: 9α,15S-dihydroxy-11-methylene-prosta-5Z,13E-dien-1-oic acid
Synonyms: 11-deoxy-11-methylene PGD2
Molecular weight: 350.5
CAS: 100648-29-1
Purity: ≥98%
Formulation: A solution in methyl acetate
Product Type|Biochemicals|Lipids|Prostaglandins||Research Area|Lipid Biochemistry|Cyclooxygenase Pathway