Description
An analgesic and antipyretic compound; an inhibitor of COX-2 that is selective over COX-1 (IC50s = 113.7 and 25.8 µM, respectively, in human blood ex vivo); induces ferroptotic cell death in primary mouse hepatocytes; has a metabolite that depletes glutathione reserves in the liver
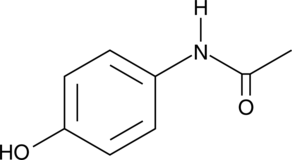
Formal name: N-(4-hydroxyphenyl)-acetamide
Synonyms: 4-Acetamidophenol|4′-Hydroxyacetanilide|APAP|NSC 109028|NSC 3991|Paracetamol
Molecular weight: 151.2
CAS: 103-90-2
Purity: ≥98%
Formulation: A crystalline solid
Product Type|Biochemicals|Small Molecule Inhibitors|Cyclooxygenases||Research Area|Cell Biology|Cell Death|Ferroptosis||Research Area|Lipid Biochemistry|Cyclooxygenase Pathway||Research Area|Neuroscience|Pain Research