Description
An irreversible, mechanism-based inhibitor of LSD1 (IC50 = 20.7 µM); irreversibly inhibits MAO-A and MAO-B (IC50s = 2.3 and 0.95 µM, respectively); PCPA inhibits nucleosomal demethylation of H3K4 (IC50 = <2 µM); induces EpiSCs to convert to more undifferentiated state but requires inhibitors of ALK5, MEK, FGFR, and GSK3 for full conversion to pluripotent mESCs; decreases proliferation of neural progenitors in the hippocampal dentate gyrus in adult mice at 10 mg/kg for ten days; decreases the time juvenile rats spend immobile in the forced swim test at 0.4-10 mg/kg
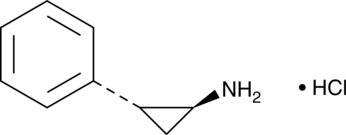
Formal name: (1R,2S)-rel-2-phenyl-cyclopropanamine, monohydrochloride
Synonyms: trans-2-Phenylcyclopropylamine|2-PCPA
Molecular weight: 169.7
CAS: 1986-47-6
Purity: ≥98%
Formulation: A crystalline solid
Product Type|Biochemicals|Small Molecule Inhibitors|Demethylases||Research Area|Cell Biology|Stem Cell Research|Differentiation||Research Area|Epigenetics, Transcription, & Translation|Erasers|Histone Demethylation||Research Area|Neuroscience|Behavioral Neuroscience|Depression