Description
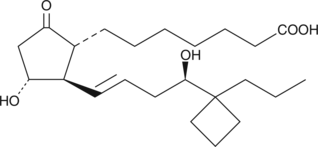
Butaprost is a structural analog of PGE2 with good selectivity for the EP2 receptor subtype. Butaprost has frequently been used to pharmacologically define the EP receptor expression profile of various human and animal tissues and cells.{3317} Serious confusion as to the structure of butaprost was generated by Gardiner in 1986,{3326} when he reported that the epimer of butaprost showing this selective activity was the C-16 (R)-epimer (See reference 2 and NOTE). In order to increase the binding affinity of (R)-butaprost for prostanoid receptors, we removed the methyl ester of (R)-butaprost and re-established the natural C-1 carboxylic acid. PG free acids generally bind to their cognate receptors with 10 to 100 times the affinity of the corresponding ester derivative. The pharmacology of (R)-butaprost has not been carefully studied, but it is generally considered to be the less active C-16 epimer.{3178} (NOTE: In the Gardiner paper in the 1986 British Journal of Pharmacology, butaprost appears on page 46 where it is given the name TR 4979. The structure as drawn is incorrect, in that the author was using and referring to the more active C-16 epimer, which is actually 16(S). The structure on page 46 shows the structure as 16(R). It was not until the late 1990’s that careful studies both in the US and Japan correctly identified the actual configuration of C-16 in the compound called butaprost is 16(S).){3326}
Formal name: 9-oxo-11α,16R-dihydroxy-17-cyclobutyl-prost-13E-en-1-oic acid
Synonyms: (±)-15-deoxy-16R-hydroxy-17-cyclobutyl PGE1|15-deoxy-16R-hydroxy-17-cyclobutyl PGE1
Molecular weight: 394.6
CAS: 215168-33-5
Purity: ≥98%
Formulation: A solution in methyl acetate
Product Type|Biochemicals|Lipids|Prostaglandins||Product Type|Biochemicals|Receptor Pharmacology||Research Area|Lipid Biochemistry|Cyclooxygenase Pathway